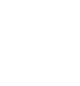Resources
A selection of resources to help you in the identification, treatment and ongoing management of people with non-tuberculous mycobacterial pulmonary disease (NTM-PD) and underlying predisposing health conditions
An evolving library of resources including videos, webinars, podcasts and articles, this section provides you with detailed information about NTM-PD and specific NTM species as well as items to support you in identifying those patients at risk of NTM and what to do when NTM is identified.

Video
BE: Webinar "Individualizing bronchiectasis therapy - let's challenge current thinking"
Duration: 60 mins
Professor James Chalmers, Professor Stefano Aliberti, Dr Eva Polverino
Recording of webinar "Individualizing bronchiectasis therapy - let's challenge current thinking" that took place on the 2nd of November 2023.

Video
BE: ERS 2023 symposium: Dr Jekyll and Mr Hyde: the two faces of bronchiectasis
Duration: 84 mins
Professor Tobias Welte, Dr Anne O'Donnell, Dr Charles Haworth, Dr Eva Polverino and Dr Pieter Goeminne
Recording of symposium "Dr Jekyll and Mr Hyde: the two faces of bronchiectasis" that took place on 10th September at ERS 2023.

Video
BE: The role of inflammation in the vicious vortex of bronchiectasis - Is it time to re-evaluate?
Duration: 54 mins
Recording of webinar on the role of Inflammation in the vicious vortex of Bronchiectasis with Prof. Stefano Aliberti, Dr Holly Keir and Dr Pieter Goeminne that took place on 8th of June 2023

Video
NTM: Imaging Webinar recording
Duration: 59 mins
Professor Rob Wilson, Professor Michael Loebinger
Recording of Insmed webinar held on the 4th May 2023 focusing on the importance of thoracic imaging in the diagnosis and management of NTM-PD

Video
BE: The role of neutrophilic inflammation in bronchiectasis
Duration: 50 mins
Professor James D Chalmers
Lecture given during WBNC 2022 conference in Prague exploring the role of inflammation in bronchiectasis

Video
BE: Webinar "Addressing the vortex of inflammation in non-CF bronchiectasis"
Duration: 66 mins
Professor Stuart Elborn
Recording of webinar "Addressing the vortex of inflammation in non-CF bronchiectasis" with Prof. Stuart Elbon, Prof. Catherine Greene, Prof. James Chalmersthat that took place on 24th May 2022.

Podcast
NTM: Convert, Cure or Fail – the treatment journey for NTM-PD
Duration: 2 mins
Prof Stefano Aliberti explains his perspectives on the outcomes of NTM-PD treatment

Podcast
NTM: Initiating treatment for NTM-PD - putting the patient at the heart of the matter
Duration: 4 mins
Prof Stefano Aliberti explains how and when to initiate treatment once NTM-PD has been identified

Podcast
NTM: Screening for NTM-PD: How to get ahead of the curve
Duration: 3 mins
Prof Aliberti discusses his approach for screening for NTM-PD

Podcast
NTM: Risks for NTM-PD that run under the radar
Duration: 2 mins
A conversation with Prof Aliberti to understand his assessment of which patients are at risk for NTM

Video
Duration: 30 mins
Professor Marc Lipman
Professor Marc Lipman discusses symptoms, diagnosis and treatment of NTM-PD with a patient

Article
NTM: Empowering the patient with NTM-PD
Read time: 4 mins
Professor Mateja Janković Makek, University of Zagreb, School of Medicine, Zagreb, Croatia

Podcast
Duration: 50 mins
Dennis Wat, Jean-Louis Herrmann, Jakko van Ingen
Liposomal Drug Delivery to manage non-tuberculosis mycobacterium pulmonary disease and other chronic lung infections.<br>James D. Chalmers, Jakko van Ingen, Roald van der Laan and Jean-Louis Herrmann.<br>Eur Respir Rev 2021; 30: 210010

Article
NTM: Understanding the risk factors that underlie NTM-PD
Read time: 12 mins
Understanding the risk factors that are common in patients with NTM-PD provides a valuable insight into patients who might benefit from testing for NTM to rule out disease and, if NTM infection is present, what might be the appropriate course of action.

Article
NTM: Importance of regular treatment monitoring for culture conversion
Read time: 8 mins
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) is complex involving multiple therapies based on the identity of the causative species and extent of disease

Article
Read time: 5 mins
The CONVERT study [NCT02344004] evaluated the efficacy and safety of amikacin liposomal inhalation suspension (ALIS) in patients with treatment-refractory NTM-PD

Article
NTM: Impact of non-tuberculous mycobacteria (NTM) on at-risk patients
Read time: 5 mins
Non-tuberculous mycobacteria (NTM) can cause serious pulmonary disease in at-risk patients, which can have a significant impact on health-related quality of life, morbidity and mortality, and increase disease progression in patients

Article
Read time: 5 mins
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) with antimicrobial agents offers the possibility of cure. In patients who meet the clinical, radiographical and microbiological diagnostic criteria for NTM-PD.

Article
Read time: 7 mins
Mycobacterium avium complex pulmonary disease (MAC-PD) is difficult to diagnose with symptoms similar to underlying lung conditions. Correct, early diagnosis and treatment are paramount to prevent disease progression.

Article
MAC: Understanding best practice in Mycobacterium avium complex pulmonary disease (MAC-PD)
Read time: 10 mins
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) varies depending on the species, extent of disease, drug susceptibility results and underlying comorbidities.

Article
Read time: 8 mins
Non-tuberculous mycobacterial pulmonary disease (NTM-PD) can be life threatening and is increasing in prevalence. International guidelines updated in 2020 provide management recommendations for the four most commonly occurring NTM pathogenic species.

Video
NTM: 2020 ATS, ERS, ESCMID, IDSA and NTM-PD Guidelines — an expert overview
Duration: 33 mins
Stefano Aliberti, Christoph Lange, Eva Polverino, Nicolas Veziris, Charles Haworth and Jakko van Ingen
Non-tuberculous mycobacterial pulmonary disease (NTM-PD) can be life threatening and is increasing in prevalence. International guidelines updated in 2020 provide management recommendations for the four most commonly occurring NTM pathogenic species.

Video
MAC: Who are the patients at risk of MAC pulmonary disease (MAC-PD)?
Duration: 3 mins
Underlying lung conditions or immunosuppression greatly increase the risk for developing MAC-PD. In this video watch and hear experts discuss who is the at-risk patient and the symptoms to be aware of.

Video
MAC: Initiation of treatment of MAC pulmonary disease (MAC-PD)
Duration: 15 mins
Knowing when to initiate treatment for MAC-PD is a multifactorial decision. In this video experts explore the rationale and timing for starting treatment.

Video
MAC: Ongoing management of MAC pulmonary disease (MAC-PD) patients
Duration: 9 mins
Once treatment is initiated, monitoring patients for a response is vital in order to plan next steps. See and hear international experts explore the key elements of ongoing treatment up to and beyond culture conversion.

Video
MAC: What to do in the event of MAC pulmonary disease (MAC-PD) treatment failure?
Duration: 4 mins
In a substantial number of patients with MAC-PD, first-line treatment with guideline-recommended triple-therapy will not provide culture conversion. In this video experts explore what options clinicians have in MAC-PD when treatment fails.

Video
Duration: 7 mins
Microbiology is pivotal to the diagnosis and treatment of MAC-PD. In this video experts share their thoughts on the role of microbiology in diagnosis, in determining effective treatment strategies and in ongoing monitoring towards culture conversion.

Video
Duration: 5 mins
In this video European experts provide their insights into the 2020 ATS/ERS/ESCMID/IDSA guidelines on NTM-PD, with a focus on MAC-PD.

Slide deck
NTM: ATS/ERS/ESCMID/IDSA NTM-PD guidelines slide deck
In this short slide deck you will find an overview of the 2020 ATS/ERS/ESCMID/IDSA guidelines.

Poster
NTM: ATS/ERS/ESCMID/IDSA NTM-PD guidelines pocket guide
In this short pocket guide find the key recommendations from the 2020 ATS/ERS/ESCMID/IDSA NTM-PD guidelines.

Article
NTM-PD at the European Respiratory Society congress 2021
Read time: 11 mins
At the virtual European Respiratory Society (ERS) 2021 held in September, non-tuberculous mycobacteria (NTM) was included in the official congress programme. NTM pulmonary disease (NTM-PD) was covered with a range of ePosters, a presentation to use

Video
NTM: www.RethinkNTM – Who, Why and When? ERS 2020 symposium
Duration: 60 mins
Watch the Insmed sponsored symposium from ERS 2020 exploring the at-risk patient for NTM-PD, the challenges of managing NTM-PD and the 2020 ATS/ERS/ESCMID/IDSA guidelines recommendations.

Video
MAC: Looking ahead - the changing landscape of MAC lung infection - WBNC symposium 2020
Duration: 65 mins
Watch the Insmed sponsored symposium at the WBNC 2020 exploring advances in management of NTM-PD, the 2020 ATS/ERS/ESCMID/IDSA guidelines recommendations and the use of ALIS in clinical practice.
The efficacy, sustainability and long-term safety of ALIS for patients with treatment-refractory MAC-PD
The CONVERT study [NCT02344004] evaluated the efficacy and safety of amikacin liposomal inhalation suspension (ALIS) in adult patients with treatment-refractory non-tuberculous mycobacterial pulmonary disease (NTM-PD) caused by Mycobacterium avium complex (MAC) in addition to oral guideline-based therapy (GBT) compared with oral GBT alone. ALIS plus oral GBT demonstrated high rates of culture conversion in 6-month data published in 2018 compared with GBT alone (29% vs 9%) and a follow-up study demonstrated culture conversions were often sustained and durable, and there were no new safety signals emerged with long-term use of ALIS.
MAC-PD is a difficult-to-treat pulmonary infection. When initial oral GBT fails, outcomes are poor and options are limited.1,2 ALIS is a novel amikacin formulation that penetrates alveolar macrophages and biofilms while limiting systemic exposure.3–5 ALIS is currently recommended by guidelines for patients with MAC-PD who fail to achieve culture conversion after at least 6 months of oral GBT in combination with oral GBT.6 ALIS has been previously tested in a Phase II study of treatment-refractory non-tuberculous mycobacterial pulmonary disease (NTM-PD) where the addition of ALIS to standard oral GBT achieved higher rates on negative sputum cultures compared with oral GBT alone.7
CONVERT was a prospective, open-label, randomised trial that evaluated the efficacy and safety of daily ALIS in addition to oral GBT in patients with refractory MAC-PD compared with oral GBT alone. A total of 336 patients with amikacin-susceptible MAC-PD and MAC-positive sputum cultures after receiving at least 6 months of oral GBT were randomised at a 2:1 ratio to receive either ALIS plus oral GBT or oral GBT alone. The primary endpoint was the proportion of patients achieving culture conversion, which was achieved if patients had three consecutive monthly MAC-negative sputum cultures by Month 6 of the study. The study was conducted in 127 centres across 18 countries in North America, Asia-Pacific and Europe. Patients were mostly female (69.3%) with a mean age of 64.7 years and many patients had underlying bronchiectasis (62.5%), chronic obstructive pulmonary disease (14.3%) or both (11.9%). The majority of patients (89.9%) were receiving GBT at enrolment, with the remainder off treatment for 3–12 months. Most patients (69.3%) were on a three-drug regimen at baseline, with 54.9% on regimens which included a macrolide, ethambutol and a rifamycin.8
Initial results of the trial demonstrated the efficacy of ALIS in addition to oral GBT in achieving culture conversion by Month 6.8 Patients treated with ALIS in addition to oral GBT were almost four times as likely to achieved culture conversion by Month 6,8 with 29% (n=65/224) of patients on ALIS plus oral GBT achieving culture conversion compared with only 8.9% (n=10/112) on oral GBT alone (P<0.0001).8,9
In a follow-up study published in 2021, patients who achieved culture conversion by Month 6 continued treatment for an additional 12 months, followed by off-treatment observation in order to assess the sustainability and durability of culture conversion. Following 12 months of post-conversion treatment, 63.1% (n= 41/65) of converters in the ALIS plus oral GBT arm and 30.0% (n=3/10) in the oral GBT alone arm achieved sustained conversion (P=0.0644). In the intention-to-treat population, which includes patients who did not culture convert, 18.3% (n=41/65) of patients in the ALIS plus oral GBT arm achieved sustained culture conversion compared with only 2.7% (n=3/10) in the oral GBT alone arm (P<0.0001). Three months following end of treatment, 55.4% (n=36/65) of ALIS plus oral GBT culture-converted patients also achieved durable culture conversion whereas no patients on oral GBT alone achieved durable culture conversion (P=0.0017). In the intention-to-treat population, 16.1% (n=36/224) of all patients on ALIS plus oral GBT achieved durable culture conversion versus no patients treated with oral GBT alone (P<0.0001).9
Re-emergence of a MAC strain with an identical genotype to the strain identified on initiation of treatment may indicate relapse, particularly if this occurs within the first 8 months of treatment. At the end of treatment, only 7.7% (n=5/65) of patients on ALIS plus oral GBT had relapsed compared with 30% (n=3/10) of patients on oral GBT alone. In contrast, recurrence of MAC after 8 months of treatment may indicate reinfection, in which MAC has been reacquired from the environment, and is treated as a new MAC infection. In total, 4.6% (n=3/65) of patients on ALIS plus oral GBT were reinfected with MAC, compared with 10% (n=1/10) of patients on oral GBT alone.9
Treatment emergent adverse events (TEAEs) occurred mainly in the first 8 months of treatment and were mainly respiratory. Respiratory TEAEs were reported more frequently in the ALIS plus oral GBT arm and included dysphonia (61.5%), cough (41.5%), dyspnoea (21.5%), and haemoptysis (20.0%). Nephrotoxicity TEAEs were balanced between the two arms (n=2 in both arms) and ototoxicity-related TEAEs in the ALIS plus GBT arm were primarily tinnitus (10.8%) and dizziness (7.7%). Only four patients discontinued treatment because of TEAEs in the ALIS plus oral GBT converter arm. Three patients who achieved culture conversion died, all in the oral GBT alone arm.9
The CONVERT study showed that the addition of ALIS to oral GBT significantly increased the likelihood of culture conversion by Month 6 compared with oral GBT alone, providing the first evidence in a randomised trial in addition to the Phase II study of efficacy against treatment-refractory MAC-PD (Figure 1).8 In addition, culture conversion in patients treated with ALIS in addition to oral GBT was generally sustained and durable with low risk of relapse (Figure 1).9 Long-term exposure to ALIS did not present any new safety concerns, with most TEAEs consistent with administration of an inhaled add-on antibiotic and generally occurring in the first 8 months of treatment.9 Overall, these results highlight the clinical utility of ALIS in the management of patients with refractory MAC-PD.
Figure 1. Proportion of patients achieving culture conversion by the first month of conversion.8,9
Month 4 was the last time point at which the first of three negative sputum cultures could be achieved for a patient to be considered a converter at Month 6.
ALIS, amikacin liposomal inhalation suspension; GBT, guideline-based therapy.
References:
- Griffith DE, Aksamit TR. Therapy of refractory nontuberculous mycobacterial lung disease. Curr Opin Infect Dis 2012;25:218–27.
- Jo K-W, Kim S, Lee JY, Lee SD, Kim WS, Kim DS, et al. Treatment outcomes of refractory MAC pulmonary disease treated with drugs with unclear efficacy. J Infect Chemother 2014;20:602–6.
- Rose SJ, Neville ME, Gupta R, Bermudez LE. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS ONE 2014:9:e108703.
- Malinin V, Neville M, Eagle G, Gupta R, Perkins WR. Pulmonary deposition and elimination of liposomal amikacin for inhalation and effect on macrophage function after administration in rats. Antimicrob Agents Chemother 2016;60:6540–9.
- Zhang J, Leifer F, Rose S, Chun DY, Thaisz J, Herr T, et al. amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol 2018;9:915.
- Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020;56:2000535.
- Olivier KN, Griffith DE, Eagle G, McGinnis JP 2nd, Micioni L, Liu K, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 2017;195:814–23.
- Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium aviumcomplex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med 2018;198:1559–69.
- Griffith DE, Thomson R, Flume PA, Aksamit TR, Field SK, Addrizzo-Harris DJ, et al. Amikacin liposome inhalation suspension for refractory Mycobacterium avium complex lung disease: sustainability and durability of culture conversion and safety of long-term exposure. Chest 2021; https://doi.org/10.1016/j.chest.2021.03.070
![]()
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Importance of regular treatment monitoring for culture conversion
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) is complex involving multiple therapies based on the identity of the causative species and extent of disease.1 Outcomes are often suboptimal and expert consultation is recommended for more complex cases.1 Regular monitoring of sputum and adverse reactions is recommended to ensure the best outcome and help achieve culture conversion.1
Guideline-based treatment recommended for NTM-PD
Treatment of NTM-PD varies depending on the species, drug susceptibility results, extent of disease and underlying comorbidities.1 Inappropriate management can lead to irreversible lung damage;2 left untreated, cavitary PD can progress rapidly to respiratory failure.3
Treatment regimens for NTM-PD comprise multiple antimicrobial agents taken for prolonged periods of time and that are frequently associated with clinically significant side-effects.1 For the best chance of success, it is imperative that patients receive the recommended guideline-based drug combinations from the outset.3 Nonetheless, outcomes are often suboptimal resulting in reinfection, and expert consultation may be required for more complex cases.1
Monitoring of causative species
Monitoring of the causative NTM species in PD is key for optimal disease management as it influences the recommended guideline-based antimicrobial treatment regimen.3 All clinically relevant NTM isolates should be identified by molecular methods throughout the course of disease management.1 Ideally samples are frozen and saved in order to distinguish reinfection from relapse if it occurs.1
Standardised monitoring of NTM-PD treatment outcomes
Clinical, radiographic and microbiological data should be collected to assess whether a patient is responding to therapy.1 The 2020 ATS/ERS/ECCMID/IDSA NTM guidelines recommend obtaining sputum specimens for culture every 1 to 2 months to determine if/when culture conversion occurs, as this often determines the length and course of treatment.1 Regular chest radiographs or chest computed tomography imaging may be beneficial for defining a radiographic response, although there can be wide variability in findings given the common occurrence of underlying lung disease.1 Both the 2020 NTM guidelines and those published by the British Thoracic Society (BTS) in 2017 highlight that frequent radiological monitoring may be of use,1,4 and the BTS guidelines suggest serological testing may also be useful for routine monitoring of patients with NTM-PD although the supporting evidence for this is currently inconsistent.4
Table 1 summarises the NTM-NET international expert consensus definitions for key outcome parameters in the treatment of NTM-PD, which if applied in clinical practice will help standardise clinical management and monitor treatment success.5 Universal application of these outcome measures will increase the quality of evidence available to support treatment regimens in difficult-to-treat NTM infections.5
Table 1. NTM-NET treatment outcome definitions for NTM-PD5
|
Treatment outcome |
Definition |
|
Culture conversion |
The finding of at least three consecutive negative mycobacterial cultures from respiratory samples, collected at least 4 weeks apart, during antimycobacterial treatment (the sampling date of the first negative culture is then the date of culture conversion) |
|
Microbiological cure |
Finding multiple consecutive negative, but no positive, cultures with the causative species from respiratory samples after culture conversion and until the end of antimycobacterial treatment |
|
Clinical cure |
Patient-reported and/or objective improvement of symptoms during antimycobacterial treatment, sustained until at least the end of treatment, but no cultures available to prove culture conversion or microbiological cure |
|
Cure |
Antimycobacterial treatment completed, with fulfilment of criteria for both microbiological and clinical cure |
|
Treatment failure |
The re-emergence of multiple positive cultures or persistence of positive cultures with the causative species from respiratory samples after ≥12 months of antimycobacterial treatment, while the patient is still on treatment |
|
Recurrence |
The re-emergence of at least two positive cultures with the causative species from respiratory samples after cessation of antimycobacterial treatment |
|
Relapse |
The emergence of at least two positive cultures with the same strain of the causative species after the end of treatment |
|
Reinfection |
The emergence of at least two positive cultures with a different strain of the causative species or a strain of a different species after the initiation of the treatment episode |
Monitoring treatment success in MAC-PD
Patients with Mycobacterium avium complex pulmonary disease (MAC-PD) should show clinical improvement within 3 to 6 months and should convert their sputum to negative within 12 months on guideline-based macrolide-containing regimens.3 Acid-fast bacilli smears and cultures of sputum should be obtained monthly during therapy to monitor for a microbiological response.3 Lack of culture conversion after 6 months of treatment is a predictor of treatment failure at 12 months.5 The 2020 NTM guidelines recommend that if sputum cultures have not converted to negative after 6 months of guideline-based treatment additional treatment strategies should be considered.1 If culture conversion is achieved, treatment and monitoring should continue for a further 12 months.1
Monitoring treatment success in Mycobacterium kansasii PD
Current rifampicin-based treatment regimens for M. kansasii PD are associated with a high rate of success.1 Treatment is recommended for a fixed duration of 12 months with culture conversion anticipated within 4 months.1 Monthly monitoring of sputum specimens is recommended throughout treatment to monitor progress; expert consultation should be sought if cultures fail to convert.1
Monitoring treatment success in M. abscessus PD
- abscessus is difficult to treat, so the 2020 NTM ATS/ERS/ECCMID/IDSA guidelines recommend expert consultation should be sought before treatment is started.1 The optimum treatment duration of M. abscessus PD is currently unknown and expert consultation can assist in determining an individualised treatment duration for a patient dependent on disease severity and response to treatment.1 The goal of 12 months of negative sputum cultures while on therapy may be reasonable, but there is no medication strategy to reliably achieve this goal.3 No antibiotic regimens based on in vitro susceptibilities have been shown to produce long-term sputum conversion for patients with M. abscessus PD.3 Symptomatic improvement, radiographic regression of infiltrates, or improvement in sputum culture positivity short of conversion may be more realistic goals of therapy.3
Monitoring treatment success in M. xenopi PD
The optimal duration of treatment for patients with M. xenopi PD is unknown, but treatment duration of less than 6 months has been associated with higher mortality and recurrence.1 Treatment outcomes generally improve if the duration of treatment increases, which outweighs the risk of adverse events associated with longer treatment.1 In patients with M. xenopi PD, the 2020 NTM guidelines recommend treatment for at least 12 months after culture conversion is achieved.1 In patients with severe disease, expert consultation is recommended.1
Monitoring for adverse reactions
The drugs used to treat NTM-PD are frequently associated with adverse reactions.1 Rapid identification and management of an adverse reaction is likely to improve the chances of treatment success.1 Educating patients regarding potential adverse reactions and monitoring for them is an important component of management.1 Monitoring should include visual acuity (ethambutol and rifabutin), red–green colour discrimination (ethambutol), liver enzymes (clarithromycin, azithromycin, rifabutin, rifampicin, isoniazid), auditory and vestibular function (streptomycin, amikacin, clarithromycin, azithromycin), renal function (streptomycin and amikacin), and leucocyte and platelet counts (rifabutin).3
Therapeutic drug monitoring
Therapeutic drug monitoring (TDM) may have a role in the treatment of NTM-PD where drug malabsorption, underdosing or clinically important drug–drug interactions are suspected.1 It could help overcome treatment failures not explained by poor adherence or drug resistance or help avoid subtherapeutic or toxic drug concentrations.1 However, NTM-PD guidelines from the British Thoracic Society suggest that TDM should not be routinely performed in patients with NTM-PD in receipt of antibiotics, apart from those on aminoglycosides.4 In patients with prescribed IV aminoglycosides serum levels and serum creatinine levels should be measured.4
Future blood-based biomarkers for monitoring treatment success
Assessing progression of disease or response to treatment remains a major challenge in the clinical management of NTM-PD.6 At present, culture of serial sputum samples serves as the primary biomarker for monitoring treatment response.6 However, a number of blood-based biomarkers are currently under examination for NTM-PD and these may offer a more practical alternative.6 Unlike sputum, blood is readily available throughout the treatment course and would support precise comparisons with baseline levels.6 Both serum anti-glycopeptidolipid immunoglobulin A and carbohydrate antigen 19-9 have shown promise but larger studies are required.6
Summary
In a long-term condition such as NTM-PD monitoring is key and includes monitoring to determine diagnosis and the best point at which to initiate therapy, monitoring to determine the point of culture conversion, monitoring post-conversion and monitoring for any adverse events related to drug therapy. Diagnosis and treatment initiation requires monitoring of clinical symptoms, radiological changes and microbiological changes with the view to start prompt therapy when the acid-fast bacilli or cavities are present.1 Treatment for NTM-PD is characteristically arduous for patients, so monthly monitoring whilst on therapy to determine the point of culture conversion is essential, enabling a move to post-culture conversion maintenance therapy or the addition of other treatment strategies. And once culture conversion is secured regular monitoring to determine patient status and to counter any re-emergence or re-infection is essential. What does the future hold for NTM-PD monitoring? Currently, no biomarker based strategies are available, but a number are in development6 which may hold promise in the future.
References:
- Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 2020;71:e1–e36.
- Basille D, Jounieaux V, Andréjak C. Treatment of other nontuberculous mycobacteria. Semin Respir Crit Care Med 2018;39:377–82.
- Griffith DE, Aksamil T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416.
- Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017;72(Suppl 2):ii1-ii64.
- Van Ingen J, Aksamit T, Andrejak C, Böttger EC, Cambau E, Daley CL, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J 2018; 51:1800170.
- Vinnard C, Mezochow A, Oakland H, Klingsberg R, Hansen-Flaschen J, Hamilton K. Assessing response to therapy for nontuberculous mycobacterial lung disease: quo vadis? Front Microbiol 2018;9:2813.
![]()
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Empowering the patient with NTM-PD
When a patient first encounters their diagnosis, it is likely that they will not know much about either non-tuberculous mycobacteria (NTM) or NTM pulmonary disease (NTM-PD). However, given the nature of the disease, expanding your patient’s knowledge about it is very important as it will allow them to take a more active role in the management of their condition.
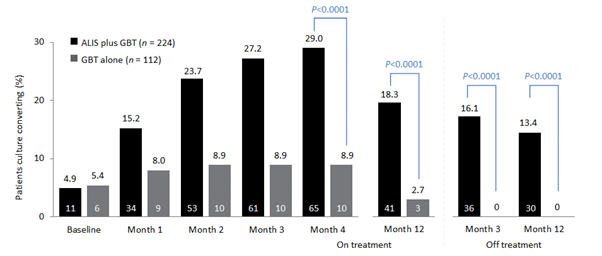
As the name itself explains, NTM are not Mycobacterium tuberculosis (MTB). Unlike MTB, NTM are not obligatory pathogens and their isolation from respiratory specimens alone is not enough to diagnose NTM-PD. In addition to NTM isolates (usually and preferably from more than one specimen), for the diagnosis of NTM-PD your patient should have clinical symptoms (respiratory or systemic) that cannot be attributed to another disease (i.e. an exacerbation of an underlying obstructive lung disease or other confirmed infection) and radiological findings consistent with NTM infection. Once the diagnostic criteria have been met and a diagnosis of NTM-PD reached the patient needs to understand that the condition is not contagious (except in very rare circumstances involving specific patient groups such as patients with cystic fibrosis). They do not have to be isolated, they do not have to be hospitalised (except in cases of severe disease and/or the need for multiple parenteral antibiotic administration) and, in fact, the decision to treat immediately is dependent on multiple factors, with immediate treatment required for severe disease and perhaps not for more mild disease.
Diagnosing NTM-PD should include NTM isolates from multiple specimens, clinical symptoms and radiological findings
NTM-PD is generally a slowly progressive disease and treatment often involves regimens with three or more antibiotics that patients need to take for more than a year (12 months after specimen culture conversion to negative; usually 16 to 18 months in total) to improve the probability of achieving cure and lessen the likelihood of recurrence. Patients need to know that current treatment regimens do not achieve complete cure in all patients despite proper treatment adherence. In general, the probability of NTM-PD cure depends on patient-specific factors (i.e. present comorbidities such as chronic lung disease), the isolated NTM species (some are tougher to eradicate because of antibiotic-resistance profiles) and the severity of the disease at presentation. Hence, upon diagnosis, the question ‘to treat or not to treat’ arises and patients should have appropriate and adequate information for them to decide.
In patients with severe forms of NTM-PD the the decision to start treatment is straightforward. For example, if the patient has radiological cavities, severe respiratory or systemic symptoms, isolation of more pathogenic NTM species or has immunocompromising conditions there is generally a clear benefit that outweighs the potential risks (i.e. drug adverse events) associated with the treatment. On the other hand, when your patient has a very mild form you, the treating physician, might likely recommend a ‘watch and wait’ strategy during this period you optimise your patient’s condition so that if treatment, if required in the future, they have the opportunity for the best possible outcome and culture conversion (Figure 1).
Figure 1. Overview of a treatment strategy for patients with NTM isolates and NTM-PD
Empowering patients with knowledge about NTM-PD is important and includes education about the disease, treatment, the need to adhere to treatment and the duration of treatment as recommended by guidelines as well as outcomes, and if possible linking them with other patients or patient groups
In some cases, however, the best approach to take is not that clear. As already stated, NTM-PD is usually a slowly progressive non-contagious disease, and the patient should have a significant role in the decision-making process. Moreover, it should be explained that an initial decision to treat or not treat can be altered down the road. For example, if the patient and you, their physician, opted for treatment and some months later no clinical (and/or microbiological and radiological) improvement is seen and/or the patient experiences severe side-effects that are not manageable or develop intolerance to drug therapy they may decide to stop treatment. However, the patient should expect their physician to encourage them to persevere if they believe that the benefits still outweigh the risks and that there is room to alter the regimen in pursuit of clinical cure. Also, if the patient decides to watch and wait, and their symptoms worsen, or they want to see whether their initial condition will improve upon treatment then the patient can talk to their physician and reconsider their initial decision (Figure 1).
The decision to start treatment or stop treatment is flexible and depends on the patient condition during a period of watchful waiting or during antibiotic treatment
Hence, education about the condition, mutual understanding and close partnership between the patient and their physician provides the basis for successful disease management. All parties must understand the importance of weighing up the benefits and risks of treatment in an individual case and recognise that the risk/benefit ratio may change over the course of treatment, necessitating reconsideration of the initial decision. In both the case of treatment and the ‘watch and wait’ strategy, close monitoring and constant partnership between the patient and their physician is fundamental for achieving the optimal outcome.
Successful management of NTM-PD requires a personal approach with an open and honest partnership between clinicians and patients with close monitoring
References:
Further reading
Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020;56(1):2000535.
Benefits of early treatment initiation in non-tuberculous mycobacterial pulmonary disease (NTM-PD)
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) with antimicrobial agents offers the possibility of cure.1 In patients who meet the clinical, radiographical and microbiological diagnostic criteria for NTM-PD, the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for NTM-PD recommend initiation of treatment rather than watchful waiting.1 Initiation is especially important in the context of positive acid-fast bacilli sputum smears and/or cavitary lung disease1as there may be an increased rate of progression and poor treatment outcomes if treatment is delayed.1
Evidence of disease progression in untreated MAC-PD
Several studies have shown that most patients diagnosed with Mycobacterium avium complex pulmonary disease (MAC-PD) have progressive disease resulting in the need for antibiotic treatment.2,3 In a recent study of 488 newly diagnosed patients at the Asan Medical Center in South Korea, 305 (62.5%) patients showed progressive MAC-PD resulting in treatment initiation within 3 years of diagnosis.2 Similarly, in another study of 40 untreated patients with the nodular bronchiectatic form of MAC-PD (most with minimal symptoms), who underwent serial chest computed tomography (CT) scans for a minimum of 4 years, 39 (97.5%) experienced disease progression with a significant increase in overall CT score.3 It is noted in the 2020 NTM-PD guidelines that some subgroups (minimal nodular/bronchiectatic disease) may be safely, but regularly, followed without antimicrobial therapy; however, those with cavity disease should always receive prompt antibiotic treatment.1
Factors influencing the decision to initiate treatment
The decision to treat may be influenced by both host factors and infecting bacterial species. Certain factors like cavitary disease and low body mass index have been associated with progressive disease and may necessitate earlier consideration of antibiotic treatment.2 In very frail patients with very mild nodular bronchiectatic disease, the balance between efficacy and tolerability may favour watchful waiting.1
The clinical relevance of NTM varies significantly between species (Figure 1) and may also differ geographically.1,4 For example, species such as M. gordonae have low pathogenicity and rarely cause disease in humans, whereas M. kansasii is highly pathogenic.1,4
Figure 1. Clinical relevance (the percentage of patients with isolates of these species that meet the ATS/IDSA diagnostic criteria) of non-tuberculous mycobacterial species. M., Mycobacterium. Adapted from Zweijpfenning (2018).5
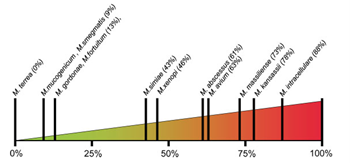
The most common NTM pathogens include MAC, M. kansasii and M. xenopi among the slowly growing NTM and M. abscessus among the rapidly growing NTM.1
Meeting the guideline-recommended diagnostic criteria for NTM-PD
Diagnostic criteria within the guideline is based on:
- Clinical symptoms e.g. worsening of symptoms of underlying lung conditions, or onset of new, persistent symptoms in patients at risk of NTM-PD e.g. haemoptysis, weight loss, fatigue
- Radiological findings on X-ray or hight-resolution CT scan such as nodular or cavitary opacities
- Microbiological findings from a) at least two expectorated sputum or b) positive culture from at least one bronchial wash or lavage or c) positive culture for NTM and biopsy from transbronchial or lung biopsy plus one or more culture positive sputa or bronchial washing.
Patients suspected of having NTM-PD who do not meet the diagnostic criteria should be actively managed and followed with serial CT scans until the diagnosis is firmly established or excluded and should start or continue recommended techniques such as airway clearance.6
The decision to initiate antibiotic treatment
NTM-PD is associated with diminished health-related quality of life that correlates with severity of lung impairment;7 antimicrobial treatment may be associated with improvement.8
NTM-PD treatment decisions are often difficult and require experience in managing the disease. This can mean that it may be necessary for a peer consultation or referral to a pulmonologist or infectious disease specialist with experience in NTM-PD.1,9 The virulence and potential for progressive disease must be evaluated once the NTM species is identified in order to determine treatment. In the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for NTM-PD for example, it is recommended that for species of low pathogenicity such as M. gordonae, treatment is only indicated if repeated positive cultures over several months are observed, along with strong clinical and radiological evidence of disease whereas in many patients only one positive M. kansasii sputum culture may be required in order to initiate treatment.1 Similarly, clinically significant MAC-PD is unlikely in patients who have a single positive sputum culture during the initial evaluation but can be as high as 98% in those with ≥2 positive cultures.1 Two or more MAC-positive cultures indicate active MAC infection requiring a treatment decision, whereas for patients identified with M. kansasii, treatment should be initiated as soon as a single positive culture is obtained.1
Regardless of the infecting organism, the decision to initiate antibiotic treatment should be individualised considering the patient’s symptoms, the pathogenicity of the organism, radiological findings, microbiological results and importantly, the patient’s wish and ability to receive treatment as well as the goals of therapy.1 Any treatment decision should include a discussion with the patient that outlines the potential side-effects of antimicrobial therapy, the uncertainties surrounding the benefits of antimicrobial therapy and the potential for recurrence including reinfection (particularly in the setting of nodular/bronchiectatic disease).1 Guidelines recommend regular sputum cultures and routine monitoring to assess disease progression.1
 Following treatment initiation, sputum specimens should be obtained for culture every 1 to 2 months to document when sputum cultures become negative and to survey for the appearance of other organisms. 1
Following treatment initiation, sputum specimens should be obtained for culture every 1 to 2 months to document when sputum cultures become negative and to survey for the appearance of other organisms. 1
Clinical and radiographical assessments should be performed alongside the microbiological assessments to determine if the patient is responding to therapy.1
Retrospective studies have shown that most patients with MAC-PD who convert on treatment do so within 6 months of starting treatment.11–13
If you decide not to initiate antibiotic treatment, an active monitoring plan is recommended by the guidelines.1 Study data suggest that untreated NTM-PD could progress.2,3
References:
- Daley Cl, et al. Clin Infect Dis 2020;71:e1–e36.
- Hwang JA, et al. Eur Respir J 2017;49:1600537.
- Park TY, et al. PLoS One 2017;12:e0185774.
- van Ingen J, et al. Thorax 2009;64:502–6.
- Zweijpfenning SMH, et al. Semin Respir Crit Care Med 2018;39:336–42.
- Lipman M, et al. BMJ Open Respir Res 2020 ;7 :e000591
- Mehta M, Marras TK. Respir Med 2011;105:1718–25.
- Czaja CA, et al. Ann Am Thorac Soc 2016;13:40–8.
- Ryu YJ, et al. Tuberc Respir Dis 2016;79:74–84.
- Lee MR, et al. Clin Microbiol Infect 2015;21:250.e1–250.e7.
- Furuuchi K, et al. Chest 2020;157:1442–5.
- Koh WJ, et al. Eur Respir J 2017;50:1602503.
- Moon SM, et al. Eur Respir J 2019;53;1801636.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
,
,
NTM-PD at ECCMID 2021
NTM-PD at ECCMID 2021
Non-tuberculous mycobacterial (NTM) infection and NTM pulmonary disease (NTM-PD) are rare diseases and have largely been overlooked in the past. It was welcome to see at the 2021 ECCMID annual congress that NTM and NTM infection/NTM-PD are emerging from the shadows with 3 symposia and more than 20 abstracts directly related to the species identification, diagnosis or treatment of NTM infection and NTM-PD. An overview of the most relevant information presented pertaining to NTM-PD specifically and NTM infection as it might influence our thinking for NTM-PD is provided here and presents an exciting snapshot into emerging scientific and clinical efforts to combat this disease.
For the first time NTM infection and NTM-PD has had a noticeable presence within a highly visible European microbiological congress. A wealth of scientific and clinical research is emerging to tackle the challenges of NTM-PD with the aim, in the future, of improving the clinical outlook for patients.
New recommendations for treating NTM-PD – the 2020 guidelines
In the “Meet The Expert session” ‘Nontuberculous mycobacterial pulmonary disease – the new ATS/ERS/IDSA/ESCMID guideline’, Dr Jakko van Ingen and Professor Claire Andrejak discussed the content to orient participants on new recommendations and answer questions from the audience.
For the first time guidelines for NTM-PD are international providing consistent evidence-based recommendations based on 22 PICO questions with graded evidence of systematic literature review.1 The limitation of the guidelines is the focus only on 4 key mycobacterial species MAC, M. kansasii, M. abscessus, M. xenopi.1 Importantly the guidelines now cover microbiological diagnostics with clear messages to obtain ≥3 respiratory samples each obtained >1 week apart, a recommendation for full speciation of the organism identified so that the clinical virulence of the infecting organisms can be determined, and clear guidelines to undertake susceptibility testing once species are identified. Dr van Ingen recommended to test and report microbiological susceptibility as per CLSI M24/M62 guidelines in broth microdilution and, if not available in regional labs, samples should be sent to reference centres. Diagnostic criteria in 2020 remain unchanged from 20072 and focus on clinical symptoms, radiological evidence and microbiological evidence of 2 positive culture of the same subspecies in order to exclude errors from a single sample.
New recommendations exhort clinicians to start treatment in patients with positive acid-fast bacilli sputum smears as this is suggestive of a high bacterial load, or if there is radiological evidence of cavitary lung disease suggestive of progressive disease.1 Possible reasons to wait to initiate treatment besides mild disease include assessing the readiness of the patient to begin an arduous treatment journey of 12 months or more, understanding of the drug susceptibility of the species identified and potential for recurrent infection.
In MAC-PD macrolides form the backbone of treatment and the 2020 guidelines suggest that azithromycin over clarithromycin should be considered, and in the absence of additional clinical data, macrolides plus ethambutol and rifampicin should be used as a 3-drug regimen;1 although Professor Andrejak outlined that studies of 2 drug regimens in MAC-PD are underway (NCT03672630). In patients with severe disease parenteral amikacin or streptomycin should be considered in the early treatment period. For those patients not culture converting by 6 months, the guidelines newly recommend the addition of Amikacin Liposomal Inhalation Suspension (ALIS) based on Phase 3 clinical data from the CONVERT study.1,3
In M. xenopi no correlation between drug susceptibility and clinical outcomes exists and so susceptibility testing is not recommended. With respect to treatment moxifloxacin or clarithromycin can be used and should be included in a treatment regimen of at least 3 drugs.1 Professor Andrejak suggested the possibility to use parenteral amikacin in M. xenopi PD, and an investigator-led study in France to explore the utility of ALIS in M. xenopi is due to start.
In M. kansasii treatment recommendations are to use 3 drug regimens of rifampicin, ethambutol and isoniazid or azithromycin for 12 months; there is no role for aminoglycosides.1 The guideline recommends susceptibility testing at baseline for rifampicin and clarithromycin, particularly given the increase in macrolide resistance. In instances of rifampicin resistance or patients intolerant to rifampicin then fluoroquinolones can be used, but this applies only to M. kansasii and not to other species.1 In patients with mild nodular disease with a macrolide-based regimen a thrice weekly dosing regimen is possible, but all regimens should be dosed for 12 months.
M. abscessus bacterial complex (MABC) -PD is one of the most difficult mycobacterial infections to treat1 but the speakers, both authors of the guidelines noted that current recommendations are relatively weak for this species due to lack of evidence. It was stressed that sub-speciation of M. abscessus is essential as M. abscessus subsp. massiliense is macrolide susceptible whilst M. abscessus subsp. abscessus may be susceptible but prone to inducible resistance. At this time the recommendation is to work closely with an expert centre for NTM-PD and treatment should focus on at least 3 drugs including amikacin, imipenem, macrolides, tigecycline and clofazimine. Use of macrolides depends on susceptibility and should not be used in cases of mutational resistance. The duration of treatment post-culture conversion is as yet unknown and the composition of long-term regimens has not been determined. Dr van Ingen highlighted the need for full clinical trials in M. abscessus rather than case series as are currently available – the medical unmet need in these patients is high and further data on appropriate therapy is needed.
Professor Andrejak counselled that continued monitoring especially microbiological evaluation of sputum every 1–2 months on treatment is essential to determine response to therapy. Similarly, monitoring for adverse events is essential and should focus on liver function tests, audiograms, ECG and so on dependent on the antimicrobials included in the treatment regimen.
Within this session, an overview of new drugs in the pipeline were presented and this demonstrates an unprecedented era of focus and development for NTM-PD. These include minocycline, tedizolid, clofazimine and ALIS that are being evaluated in the laboratory, dynamic models such as hollow fibre models and early human Phase 1/Phase 2 studies.
Meeting the challenges of NTM organisms and NTM-PD
The symposium ‘NTM-PD: do we need to rethink its management’ chaired by Dr van Ingen and Dr Daniela Cirillo (sponsored by Insmed) explored the challenges mycobacterial pulmonary infection present, that requires a new way of thinking for management.
NTM present a particular challenge to treatment because of their cellular physiology including hydrophobic, thick cell walls and their ability to sequester in intracellular spaces including phagocytic cells and biofilms.4–7 Professor Matteo Bassetti presented an overview of sequestration into intracellular spaces and how NTM species, such as M. avium, manipulate normal macrophage processes to reduce phagosome-lysosome fusion, up-regulate genes to facilitate MAC replication and reduce macrophage function so that macrophage apoptosis is controlled enabling effective release of MAC bacteria into the lung environment and infection of neighbouring macrophages so driving a cycle of infection.8–10 Similarly, incorporation of NTM organisms into biofilms presents a physical challenge to the host and to antimicrobial entry and biofilms persist following initiation of phagocyte apoptosis to arrest normal biofilm breakdown mechanisms.11
The problem of the mycobacterial physiology is also coupled with ubiquitous distribution in the environment as presented later in the symposium by Professor Veziris.12,13 NTM-PD is largely initiated by inhalation of organisms in patients with underlying risk factors or may be aspirated from the gastrointestinal tract. Once in the lung NTM can evade antimicrobial action as lung penetration of many systemically administered antibiotics is limited14 requiring high doses to achieve sufficient lung concentrations which may not be possible due to side effects.15 Penetration of many antibiotics into intracellular spaces such as macrophages and biofilms is also poor.14–16
Despite ubiquitous distribution of NTM organisms, exposure does not equate to universal infection. Rather a series of underlying risk factor predispose the tipping point from exposure to infection including underlying lung conditions and some patient morphological characteristics.17 Similarly, diagnosis of NTM-PD or MAC-PD in a patient may not lead to immediate treatment as there are factors of spontaneous culture conversion,18 patient comorbidities and patient wishes to consider.
Aerosolised inhaled antibiotics may address the problem of lung penetration and may reduce selection pressure for multi-drug resistant organisms but is unable to address the issue of macrophage or biofilm penetration providing a rationale for liposomal encapsulation. Liposomes provide an opportunity to penetrate cell membranes, to improve pharmacokinetics of encapsulated antibiotics and potentially reduce systemic toxicity.19 ALIS) licensed in Europe as ARIKAYCE® liposomal 590 mg nebuliser dispersion, is the first inhaled liposome encapsulated antibiotic to be approved and is indicated for use in adult patients with MAC-PD who have limited treatment options and do not have cystic fibrosis, in consideration of official guidance on the appropriate use of antibacterial agents.20 Early studies have demonstrated effective deposition in the lung post-inhalation that persists over 24 hours, and effective penetration in an in vitro study of both MAC infected macrophages and biofilms.21,22
For MAC-PD treatment is lengthy and relies on a macrolide backbone of azithromycin plus ethambutol and rifampicin for at least 6 months to secure culture conversion and then 12 months beyond.1 Professor Veziris presented data to support a new recommendation in the guideline, that of prescribing ALIS to patients with MAC-PD who fail to culture convert by 6 months. A Phase 3 study has demonstrated that using ALIS in patients who have failed oral guideline-based therapy (GBT), many of whom had refractory disease for many years, provides culture conversion in 29% of patients compared with GBT alone 8.9% (p<0.0001);3,23 and it is in these data that guidelines have been revised for patients with MAC-PD (Figure 1).1 Professor Veziris presented further data from ALIS from the long-term follow-up phase of the Phase 3 study which demonstrates that culture conversion is durable while patients are on ALIS plus GBT therapy and is sustained for 3 months or more once all antimicrobial therapy is removed.23
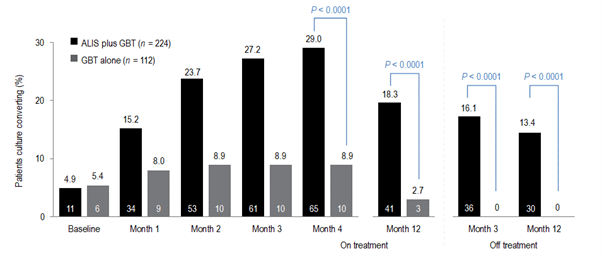
Figure 1. Proportion of patients achieving or maintaining culture conversion
Month 4 was the last time point at which the first of three negative sputum cultures could be achieved for a patient to be considered a convertor at month 6.
GBT, guideline-based therapy.
Emerging technologies and treatments in NTM-PD
The symposium ‘What’s new in mycobacterial disease’, chaired by Professor Florian Maurer and Professor Thomas Schön, explored a range of new developments in NTM-PD and NTM infection. The symposium included two presentations that have the future potential to impact clinical management, one by Dr van Ingen to explore a biomarker to predict treatment success in NTM-PD and one about the potential activity of pentamidine in MAC and M. abscessus from Professor Jelmer Raaijmakers.
Biomarkers in NTM-PD have potential to provide insight into when is the best time to treat patients with disease, the impact of treatment and determining treatment success. Dr van Ingen presented data of a biomarker that can aid prediction of culture conversion in patients once treatment is initiated. Treatment regimens for NTM-PD are often hampered by a limited evidence base and a poor rate of culture conversion despite aggressive treatment.24–26 Time to positivity for MAC organisms in sputum culture was presented as a possible tool to predict patients who will respond to treatment that could be useful in clinical practice and in clinical trials to evaluate new therapies.
The Mycobacterium Growth Indicator Tube (MGIT™) is an automated liquid culture system.27 Using sputa from 49 patients the time to positivity (TTP) in the MGIT system was explored as a biomarker for treatment response. All patients had macrolide-sensitive MAC-PD and TTP was correlated with actual clinical outcomes of conversion, defined as 2 consecutive negative cultures collected ≥4 weeks apart. Mean baseline TTP was higher in patients who culture converted than those who did not (7.68 ± 4.64 vs 4.87 ± 2.20 days, p=0.031), and TTP was also significantly different for patients with nodular-bronchiectatic disease and those with fibrocavitary disease (8.86 ± 5.62 vs 5.29 ± 1.65 days, p=0.010). Differences in TTP increased over time so that, at 3 months, TTP for those converting was 36.38 ± 12.30 days compared with 9.75 ± 5.19 days in non-convertors (p<0.001). These data suggest that MGIT TTP obtained at baseline and at 3 months provides a prediction of culture conversion for MAC-PD. However, Dr van Ingen was keen to outline that time to positivity is predictive of culture conversion only and cannot predict treatment and patient outcomes. However, the use of an early and easily available biomarker that can predict patients who are most likely to convert with therapy can be extremely helpful in planning treatment strategies for individual patients.
New therapies in NTM-PD
Novel treatment approaches were also a focus of NTM abstracts. One by Kan et al.28 suggested that the ligase PafA in the pup-proteosome system (PPS) which is essential for maintaining bacterial persistence in macrophages might provide a potential drug target for patients with persistent intracellular NTM infection. Using proteomic analysis three PafA inhibitors were identified and demonstrated reductions in intracellular mycobacterium in vitro in macrophages. The inhibitors discovered require more investigation but provide an interesting potential adjunct for treating mycobacterial infections such as NTM-PD.
ALIS as a liposomal formulation has been demonstrated in vitro to penetrate macrophages and biofilms where MAC organisms typically sequester to evade host defences and antimicrobial therapy.22 The study by Le Moigne et al.29 explored the ability of ALIS to penetrate phagocytic cells where MABC organisms reside. In this study, access to intracellular mycobacteria was explored using confocal microscopy to observe potential co-localization of ALIS and MABC in cells including epithelial cells and macrophages, and to explore intracellular antimicrobial activity. Confocal microscopy demonstrated that fluorescently tagged ALIS co-localises with MABC within a range of cells, not just phagocytic ones such as macrophages but also epithelial cells, an effect that was not observed with water soluble amikacin. Within cells ALIS demonstrated intracellular bactericidal at concentrations of 32 and 64 μg/mL at 3- and 5-days post-infection. Together, these data suggest that ALIS provides potential in MABC infection with an ability to reach intracellular spaces and have an antimicrobial action on MABC.
M. kansasii pulmonary disease is a common disease-causing mycobacterium second only to MAC and is associated with a poor outlook – with mortality rates up to 50% in patients co-infected with HIV.30 A study by Munoz-Munoz et al.31 explored the susceptibility profile of beta-lactams. Beta-lactam antibiotics are not typically used for mycobacterial infections due to the presence of constitutive beta-lactamases, but in this study beta-lactams in combination with clavulanate did demonstrate potency against the M. kansasii strain ATCC although less than with guideline-based antimicrobial therapy. Amoxicillin/clavulanate was the most active combination (MIC 8 mg/mL) but carbapenems even in the presence of clavulanate had no activity.
Emerging approaches to treating NTM-PD are the development of new molecules or understanding how older molecules can be repurposed. Professor Raaijmakers presented an interesting study exploring the use of pentamidine.32 Pentamidine is most used as an inhaled antibiotic for the treatment of pneumocystis pneumonia and Professor Raaijmakers presented data of pentamidine in isolate models to understand isolate susceptibility, intracellular penetration and efficacy against isolates in phagocytic cells and efficacy against isolates in an ELF model. In vitro time-kill assays of isolates of M. tuberculosis (n=6), M, abscessus (n=3) and M. avium (n=4) demonstrated greatest efficacy of pentamidine against M. tuberculosis at 0.5 MIC, with efficacy against M. avium at 2 x MIC but very limited response against M. abscessus with regrowth observed even at concentration of 32 x MIC.32 In vivo time-kill assay in human blood mononuclear cells (HBMCs) suggested that pentamidine was comparably effective against M. tuberculosis and M. avium with an ability for intracellular penetration but had very limited activity against M. abscessus. In hollow fibre models that emulate the ELF environment pentamidine plus a GBT regimen of azithromycin, ethambutol and rifampicin reduced bacterial density both extracellularly and intracellularly more than GBT alone, but initial reductions provided by pentamidine were not sustained and within 2–3 weeks bacterial densities in both groups were comparable.32
Improving our understanding of the mechanism of infection of MAC
Dr van Ingen’s group presented an abstract33 that explored the interplay between M. avium phagocytosed into human monocytes and clarithromycin. Post-phagocytosis upregulation in genes related to cytokine signalling and immune activation was evident in macrophages whilst within M. avium genes related to nitrate respiration and coding for M. avium antigens were upregulated. These data highlight that the host environment can greatly influence the efficacy of macrolides such as clarithromycin.
Understanding NTM infection and NTM-PD in areas of high TB
Risk factors for NTM-PD such as underlying lung disease are widely recognised, but an abstract from Cruz et al.34 presented the cases of two patients presenting with symptoms that were assumed to be tubercular given the setting of endemic TB in the country. Only on post-mortem of one patient and sputum testing of the other was NTM infection identified. Whilst these cases are in disseminated NTM infection they provide an insight into countries where TB is endemic to continue to keep NTM infection, NTM-PD and NTM testing front of mind.
As with risk factors, the geographical diversity of NTM species is well known.35 a study from Nigeria,36 a country of endemic TB and high HIV, has explored the species variation across 167 participant sputum samples. In this study in patients with HIV enrolled at a national TB clinic the predominating species was M. intracellulare (45.1%), M. interjectum (16.1%) and M. malmoense (12.9%); M. avium was identified in only 6.5% of samples and 12.9% of samples could not be speciated. These data indicate that NTM-PD infection among people infected with HIV is high, which reflects the similar historical perspective of Western countries before the advent of fully accessible high active anti-retroviral therapy (HAART).
A third study by Fraile Torres et al.37 reminds us that in many parts of the world NTM are overtaking TB as an infecting mycobacterial species. In this study 50,728 sputum samples from 15,931 patients were retrospectively examined for NTM over ten years (2010–2020). Of these isolates 3,328 samples from 1,223 patients were positive for NTM. MABC (M. abscessus subsp. abscessus, M. abscessus subsp. Massiliense, and M. abscessus subsp. bolletii ) was the most common infecting organism and among these patients an equal percentage had the underlying risk factors of NCFBE or CF (34.88%), and a small minority had a history of previous TB.
Translating NTM-PD guidelines into routine practice
Understanding drug susceptibility for any infection is important, and 2020 NTM-PD guidelines recommend specific susceptibility testing depending on the predominating infecting NTM species.1 The antimicrobial susceptibility of a range of slow growing mycobacteria were evaluated by Hunkins et al. in the USA.38 In this study of 10,668 isolates (85.2% of which were respiratory; 5 MAC species, 6 other slow growing NTM) it was noted that susceptibility to macrolides, including clarithromycin was high and consistent among species as was susceptibility to rifabutin except for M. asiaticum and M. simiae where susceptibility was approximately 60% or less. Based on the susceptibility breakpoint for IV amikacin, Susceptibility to amikacin was lower at 76.62% for M. avium and 72.44% for M. intracellulare and given the position of IV amikacin in the treatment of MAC suggests that comprehensive antibiograms may be useful to guide therapy for patients.
In a second study,39 the pattern of susceptibility of MAC isolates was explored. Using MALDI-TOF analysis of 737 strains of MAC in sputum M. avium was the most commonly identified single species (n=351, 47.62%) followed M. intracellulare/M. chimaera (combined n=386, 52.37%). Susceptibility against a range of antibiotics recommended by guidelines1 was explored. It was found that susceptibility of M. avium to clarithromycin was maintained in 95.7% of isolates, but only 3% of isolates were susceptible against ethambutol and even lower for IV amikacin. By contrast, susceptibility for these drugs against M. intracellulare/M. chimaera were better.
A cautionary abstract from India40 demonstrated that disease-driving species differ across the world and that drug susceptibility also varies greatly. In this study, the predominant species causing pulmonary disease were MABC, M. fortuitum and, to a limited extent, M. chelonae. Isolates of M. abscessus were susceptible to clarithromycin but only after extended exposure and there were marked decreases in the susceptibility patterns of isolates to imipenem, cefoxitin and fluoroquinolones compared with those reported from other countries. These data suggest that speciation is vital, and in low- or middle-income countries, infection control measures require improvement. The study authors also suggest that NTM-PD guidelines whilst valuable may not always be applicable across all countries.
In summary
At ECCMID 2021 it was clear that NTM-PD as a rare disease is emerging from the shadows with burgeoning research emerging that gives insight into future diagnostics, prognostics and treatment
References:
- Daley CL, et al. Eur Respir J 2020;56:2000535
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Griffith DE, et al. Am J Respir Crit Care Med 2018;198:1559–69.
- Chakraborty P, Kumar A. Microbiol Cell 2019;6:105–22.
- Sousa S, et al. Int J Mycobacteriol 2015;4:36–43.
- Awuh JA, Flo TH. Cell Mol Life Sci 2017;74:1625–48.
- Ganbat D, et al. BMC Pulm Med 2016;16:19.
- Sturgill-Koszycki S, et al. Science 1994;263:678–81.
- Chiplunkar SS, et al. Future Microbiol 2019;14:293–313
- Lee KI, et al. Scientific Reports 2016
- Rose SJ, Bermudez LE. Infect Immun 2014;82:405–12.
- Lee E-S, et al. J Microbiol Biotechnol 2008;18:1207–15
- Nishiuishi Y et al. CID 2007;45:347-351
- Honeybourne D. Thorax 1994;49:104–6
- Wenzler E, et al. Clin Microbiol Rev 2016;29:581–632
- Greendyke R, Byrd TF. Antimicrob Agents Chemother 2008;52:2019–26
- Prevots DR, Marras TK. Clin Chest Med 2015;36:13–34
- Hwang JA, et al. Eur Repir J 2017;49:1600537.
- Chalmers JD, et al. Eur Respir Rev 2021;30:210010.
- ARIKAYCE liposomal 590 mg nebuliser dispersion. EU Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/arikayce-liposomal-product-information_en.pdf [Accessed September 2021]
- Olivier KN, et al. ATS Congress 2016, San Francisco, CA, USA. Poster A3732.
- Zhang J, et al. Front Microbiol 2018;9:915.
- Griffith DE, et al. Chest 2021;160:831–42Apr 19:S0012-3692(21)00703.
- Kwak N, et al. ERJ 2019; 54:1801991
- Zweijpfenning S, et al. Respir Med 2017;131:220–224.
- Griffith DE, et al. Am J Respir Crit Care Med 2015;192:754–60
- Danho R, et al. ECCMID Congress 2021, virtual. Abstract 02527
- Kan HL, et al. ECCMID Congress 2021, virtual. Abstract 00910.
- Le Moigne V, et al. ECCMID Congress 2021, virtual. Abstract 00866.
- Marras TK, et al. Am J Respir Crit Care Med 2004;170:793–98.
- Munoz-Munoz L, et al. ECCMID Congress 2021, virtual. Abstract 02674
- Raaijmakers J, et al. ECCMID Congress 2021, virtual. Abstract 04116.
- Schildkraut J, et al. ECCMID Congress 2021, virtual. Abstract 03747.
- Cruz MG, et al. ECCMID Congress 2021, virtual. Abstract 00169.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Olayinka A, et al. ECCMID Congress 2021, virtual. Abstract 00942.
- Fraile Torres AM, et al. ECCMID Congress 2021, virtual. Abstract 04043.
- Hunkins J, et al. ECCMID Congress 2021, virtual. Abstract 02793.
- Fernandez-Pittol M, et al. ECCMID Congress 2021, virtual. Abstract 02394.
- Irfana M, et al. ECCMID Congress 2021, virtual. Abstract 02548.
![]()
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
An overview of the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for the treatment of non-tuberculous mycobacterial pulmonary disease
Non-tuberculous mycobacterial pulmonary disease (NTM-PD) can be life threatening and is increasing in prevalence. International guidelines updated in 2020 provide management recommendations for the four most commonly occurring NTM pathogenic species.
Non-tuberculous mycobacteria (NTM) are ubiquitous in the environment.1 The clinical presentation of NTM infection is most often pulmonary disease (PD), and rates are highest in elderly people, and in those with underlying structural airway disease, cancer or immunodeficiencies.1,2 Diagnosis and treatment of NTM-PD can be difficult2 and its prevalence is growing.1 Published in 2020, ATS/ERS/ESCMID/IDSA jointly sponsored the development of a guideline updating management recommendations for NTM-PD in adults.1 Recommendations for diagnosis and treatment of the most common pathogenic NTM species and the perspectives of international thought leaders on guideline recommendations are summarized here. The appearance of new guidelines in 2020 was widely welcomed by NTM experts.
“These guidelines represent an important achievement after 13 years with a rigorous, great methodology, starting from 22 PICO [Population, Intervention, Comparator and Outcome] questions addressed in these guidelines resulting in 31 recommendations touching the management of patients with NTM, including Mycobacterium avium complex, M. kansasii, M. xenopi or M. abscessus. The task force was composed of representatives from four different international societies, two from the US and two from Europe plus patient representatives, so a very important document in 2020”. Stefano Aliberti, University of Milan, Italy
Diagnosis
The ability of NTM to cause disease differs between species, with M. xenopi, M. kansassi, M. abscessus and Mycobacterium avium complex (MAC) of which M. avium and M. intracellulare are the most common species being responsible for most NTM-PD.1 It is clear from experts that awareness of NTM remains relatively low so understanding which patients might be at risk of NTM-PD can be suboptimal.
“Women are usually more affected than men and of course a number of patients with particular conditions like cystic fibrosis [and], or some kind of immunosuppression”. She went on to say that “It is difficult for many people to understand what patients should be tested for NTM, and when and for these you should be aware about compatible signs and symptoms. For example, persistent, long-term dry cough or over-productive cough is one of the most common symptoms, as is loss of weight, some mild fever at night and sweating”. Eva Polverino, Vall d’Hebron Hospital, Spain
Diagnosis relies on clinical, radiographical and microbiological data — laboratory identification to a species or subspecies level is key both in diagnosis and treatment decisions.1
The four most common pathogenic species are Mycobacterium avium complex (MAC), M. kansasii, M. xenopi and M. abscessus.1,3
- Clinical signs of NTM-PD include cough, sputum production, haemoptysis, dyspnoea chest pain, malaise, weight loss and night sweats.3
- Radiological signs are nodular or cavitary opacities on chest radiograph, or bronchiectasis with multiple small nodules and tree-in-bud pattern on high-resolution computed tomography scan.1
- Microbiological confirmation (one of the following) is obtained from:1
- positive culture results (same NTM species) from at least two sputum samples
- positive culture results from at least one bronchial wash or lavage
- transbronchial or other lung biopsy with mycobacterial histological features (granulomatous inflammation or acid-fast bacilli) and, either
- positive culture for NTM, or
- one or more sputum or bronchial washings culture positive for NTM.
Treatment
The recent guidelines recommend treatment initiation rather than watchful waiting on diagnosis of NTM-PD, but drug therapy should follow a careful discussion of risk–benefit with the patient.1 Treatment varies according to the species, extent of disease, drug susceptibility results and the patient’s underlying comorbidities.1 Regimens often require administration of multiple agents associated with clinically significant adverse events for a prolonged period, outcomes are often suboptimal and reinfection common. Expert consultation is often helpful.1
Guideline recommendations are made in several scenarios for patients infected with MAC, M. kansasii, M. xenopi or M. abscessus.1 However, before treatment is initiated understanding drug susceptibility for those drugs being considered for treatment is important.1
“The new guidelines do provide recommendations on drug susceptibility testing and they state that drug susceptibility testing should be performed before initiating treatment for MAC pulmonary disease for example. Importantly they state so for 2 drugs or groups of drugs, for macrolides and for amikacin. Testing amikacin susceptibility is very important because amikacin, as an IV drug, may be required in the initial phase of treatment in patients with very severe disease and in patients with refractory disease”. Jakko van Ingen, Radboud UMC, the Netherlands
Similarly, for M. abscessus susceptibility testing for macrolides and amikacin is also recommended, whilst for M. kansasii the guideline recommends susceptibility testing for rifampicin.1 For M. xenopi the guideline indicates there is insufficient evidence to recommend any specific susceptibility testing.1
MAC1
Macrolide-susceptible MAC-PD1
- A three-drug regimen including a macrolide is suggested in preference to a two-drug regimen that includes a macrolide and is recommended over a three-drug regimen with no macrolide.
- Macrolide susceptibility consistently predicts treatment success; regimens with no macrolide are associated with reduced rates of negative sputum-culture conversion, and with higher mortality.
- Three-drug regimens are recommended owing to a lack of evidence for the relative risk of macrolide-resistant MAC developing with two-drug versus three-drug regimens.
- In patients with non-cavitary nodular/bronchiectatic disease, administration of a macrolide-based regimen three times per week is preferable to daily administration.
- Intermittent and daily therapies have similar sputum conversion rates, but intermittent treatment is better tolerated and not known to be associated with development of macrolide resistance.
- In patients whose therapy has failed after at least 6 months of guideline-recommended oral guideline-based treatment, once-daily Amikacin Liposome Inhalation Suspension (ALIS) should be added to the treatment regimen.
- After culture conversion, patients should continue treatment for at least 12 months.
- This is based on the 2007 guideline recommendation (12 months of negative sputum cultures), and a lack of data on optimal duration of therapy.3
Newly diagnosed macrolide-susceptible MAC-PD1
- It is suggested that azithromycin-based, rather than clarithromycin-based, regimens are used and that initial treatment should not include inhaled amikacin (parenteral formulation) or ALIS.
- Clarithromycin and azithromycin have equal efficacy, but azithromycin is better tolerated, with fewer drug interactions, lower pill burden and is taken once daily.
Cavitary or advanced/severe bronchiectatic or macrolide-susceptible MAC-PD1
- A macrolide-based regimen administered daily rather than three times per week is suggested.
- No randomised trials have evaluated the the risk of macrolide resistance associated with intermittent versus daily regimens, so a daily regimen is preferred.
Cavitary or advanced/severe bronchiectatic or macrolide-resistant MAC-PD1
- Parenteral amikacin or streptomycin is suggested as part of initial treatment.
- Parenteral aminoglycoside is among very few options for ‘intensifying’ standard oral therapy in MAC. Administration of aminoglycoside for at least 2–3 months balances associated risks and benefits.
M. kansasii1
Rifampicin-susceptible M. kansasii-PD1
- A regimen of rifampicin, ethambutol and either isoniazid or a macrolide is suggested instead of a fluoroquinolone. It is also suggested that neither parenteral amikacin nor streptomycin be used routinely. Patients should be treated for at least 12 months.
- Isoniazid is widely used with rifampicin and ethambutol, with good outcomes. Two studies substituting isoniazid with clarithromycin also showed good treatment outcomes. There is less experience for substitution with a fluoroquinolone, but this can be used in cases of antibiotic intolerance or rifampicin resistance.
- Apart from in severe disease or where rifampicin-based regimens cannot be used, the risk of adverse reactions counts against parenteral amikacin or streptomycin.
- Daily administration is suggested for all patients receiving rifampicin, ethambutol and either isoniazid or a macrolide.
- Patients with non-cavitary nodular/bronchiectatic disease receiving the macrolide-containing regimen can also be treated three times weekly.
M. xenopi1
All patients1
- A daily treatment regimen of at least three drugs is suggested: rifampicin, ethambutol and either a macrolide and/or a fluoroquinolone (e.g. moxifloxacin). It is suggested that treatment continues for at least 12 months after culture conversion.
- In cavitary or advanced/severe bronchiectatic disease, adding parenteral amikacin to the treatment regimen and obtaining expert consultation are suggested.
- On current evidence, patients should be treated aggressively given the high mortality associated with xenopi-PD.
M. abscessus1
All patients1
- A regimen of at least three drugs is suggested (selection guided by in vitro susceptibility)
- The disease can be life threatening. Treatment regimens should be designed with expert guidance in particular with respect to treatment duration.
Strains without inducible or mutational resistance1
- A macrolide-containing multidrug treatment regimen is recommended.
- In vitro macrolide-susceptibility testing is important.
Strains with inducible or mutational macrolide resistance1
- It is suggested that a macrolide can be included for its immunomodulatory properties but is not considered part of the antibiotic regimen.
“In addition to antibiotic therapy close monitoring of the patient and patient education is very important. One of the major mistakes done in the management of patients with Mycobacterium avium pulmonary disease or any other NTM pulmonary disease, is the lack of proper monitoring, for example monthly sputum collection for microscopy culture. Also the weight of a patient and symptoms, systems recording like sputum production or fatigue recording are important as part of the monitoring of a patient during the treatment”. Christoph Lange, Research Center Borstel, Germany
The guidelines published in 2020 are very welcome in this often overlooked area of respiratory medicine. However, it is clear that treatment for patients with NTM-PD remains arduous and lengthy with multiple drug regimens that are required for 12 months or more post-culture conversion.
Abbreviations
ALIS, Amikacin Liposomal Inhalation Solution; ATS, American Thoracic Society; ERS, European Respiratory Society; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; IDSA, Infectious Diseases Society of America; MAC, Mycobacterium avium complex; NTM, Non-tuberculous mycobacteria; NTM-PD, Non-tuberculous mycobacterial pulmonary disease; PD, pulmonary disease.
References:
- Daley CL, et al. Eur Respir J 2020;56(1):2000535.
- Ratnatunga CN, et al. Front Immunol 2020;11:303.
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Impact of non-tuberculous mycobacteria (NTM) on at-risk patients
Non-tuberculous mycobacteria (NTM) can cause serious pulmonary disease in at-risk patients, which can have a significant impact on health-related quality of life, morbidity and mortality, and increase disease progression in patients with structural lung diseases. Understanding who is at risk can facilitate earlier diagnosis and treatment, which is crucial in preventing disease progression and lung function decline.
Non-tuberculous mycobacteria (NTM) are opportunistic infections that can cause infection at a wide range of body sites in patients who have underlying disease or are immunosuppressed.1 Inhalation of some NTM species in vulnerable people can cause non-tuberculous mycobacterial pulmonary disease (NTM-PD)2. NTM-PD can be caused by a variety of mycobacterial species, the most common of which is the Mycobacterium avium complex (MAC), which comprises two main species M. avium and M. intracellulare. In one study of 62 centres in 30 countries of 18,418 isolates MAC-PD accounted for 47% of incidences of NTM-PD.3
How does NTM-PD have an impact on quality of life?
NTM-PD can be a significant burden on patients. Patients with NTM-PD, including MAC-PD, may have reduced lung function, increased morbidity and mortality, and reduced health-related quality of life (HRQoL) compared with the general population.3,4–11
All-cause mortality in patients with NTM-PD can be up to four times higher than the general population, independent of other factors.10–12 For MAC-PD, studies showed a pooled estimate of five-year all-cause 5-year mortality of 27%.2 NTM-PD can also cause a significant reduction in patients’ lung function.7–9 Patients with NTM-PD have been shown to experience a more substantial reduction in forced expiratory volume in 1 sec (FEV1) compared with those without NTM-PD.8 In one study where patients with mild disease were considered not to require treatment, chronic NTM infection caused a substantial decline in lung function over time.7 NTM-PD is associated with a lower HRQoL compared with the general population, with these patients demonstrating higher scores using the St. George’s Respiratory Questionnaire (SGRQ) and lower scores using the Medical Outcomes Study 36-item Short Form Survey (SF-36).4,13 In one study, SGRQ scores in patients with NTM-PD were over 25 points worse compared with normal values.13 Another study showed that patients eventually requiring treatment for their NTM-PD had worsening SGRQ scores, suggesting an association between disease progression and lower HRQoL.4
Who is most at risk of NTM-PD?
Understanding who is at increased risk of NTM-PD can help in early recognition and diagnosis of disease. High-risk groups include tall, elderly women with a low body mass index (BMI) and abnormalities of the skeleton for example, conditions such as abnormal spinal curvatures (scoliosis, kyphosis) and structural abnormality of the chest where the sternum is pressed inward (pectus excavatum) – so-called Lady Windermere syndrome – patients with underlying lung conditions such as bronchiectasis and chronic obstructive pulmonary disease (COPD), and immunocompromised and immunosuppressed patients; exposure to NTM species is much more likely to cause disease in these groups.6,14–16
Patients with low BMI
There is an association between NTM-PD and marfanoid characteristics of elderly female patients who are taller than average with low body weight, as well as those with thoracic skeletal abnormalities; low body weight alone increases risk of NTM-PD by three-fold and thoracic abnormalities five-fold.17,18 In patients with NTM-PD, low BMI (<18.5 kg/m2) has been associated with the presence of multiple NTM isolates as well as a lower chance of treatment success.4,5 In addition, patients with lower BMI are more likely to fail treatment for their NTM-PD.4
Transplant recipients
NTM can also cause disease in immunosuppressed patients who are recipients of organ or stem cell transplants.6 Rates of NTM infections in lung transplant recipients are particularly high, and have been shown to increase post-transplant mortality, with an estimated 5-year mortality of 50%.6
Patients with structural lung diseases
NTM can cause pulmonary disease in patients with pre-existing underlying lung conditions, such as those with bronchiectasis (44.0–187.5 increased risk) and COPD (2.0–10.0 increased risk).15,16 Infection and inflammation caused by NTM in such patients can lead to deterioration of pulmonary function, causing faster disease progression compared with patients without NTM-PD.5 In one study, the presence of multiple NTM isolates in patients with COPD has been associated with a greater decline in FEV1 as well as an increase in exacerbations requiring hospitalisation compared with the absence of isolates.5
Act now for your at-risk patients
Early diagnosis and treatment of NTM-PD is crucial in preventing disease progression and declining lung function.19
NTM-PD is often misdiagnosed or diagnosed late, as symptoms of NTM-PD are similar to those of coexisting lung disease and may be present for more than 10 years before diagnosis; increasing testing for your at-risk patients may lead to a higher chance of spotting NTM-PD early.20,21 In a survey of 280 hospital-based physicians, the majority perceived NTM-PD as a significant factor for worsening respiratory function, increasing morbidity and hospitalisation in patients with bronchiectasis, although fewer perceived NTM-PD as having a significant impact on mortality.22 However, the mortality rate for NTM-PD and in particular MAC-PD is high (up to 27%),2 so recognising the risk of NTM-PD and testing your at-risk patients is important, as missing a diagnosis can lead to worse long-term outcomes for your patients, including increased risk of death.
At-risk patients with underlying lung conditions such as COPD or bronchiectasis may receive long-term macrolide monotherapy to prevent exacerbations of underlying disease.23 One study indicated that 42% of patients with bronchiectasis received macrolide monotherapy, however, macrolide monotherapy is not recommended in patients with NTM-PD because of the increased chance of macrolide resistance.22
Guidelines for managing bronchiectasis outline that any patient being considered for macrolide therapy for exacerbations should be screened for underlying NTM to rule out infection and protect antimicrobial susceptibility for NTM therapy.23
Currently, 68% of healthcare professionals do not test their patients for NTM-PD prior to initiating macrolide treatment, despite 87% perceiving these patients to be at particular risk of NTM infection.22 In fact, macrolide monotherapy is one of the major predispositions for macrolide-resistant MAC,24 so testing your at-risk patients for NTM-PD prior to initiating macrolide monotherapy is essential to reduce the emergence of macrolide-resistant NTM-PD and to increase the chance of cure. In patients with macrolide-resistant NTM-PD, treatment outcomes are poor, with an estimated all-cause 5-year mortality rate of 47%.25
References:
- Centers for Disease Control and Prevention. Nontuberculous mycobacteria (NTM) infections. https://www.cdc.gov/hai/organisms/nontuberculous-mycobacteria.html [Accessed March 2021]
- Diel R, et al. BMC Infect Dis 2018;18:206.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Kwak N, et al. BMC Pulm Med 2020;20:126.
- Huang CT, et al. Int J Tuberc Lung Dis 2012;16:539–45.
- Friedman DZP, et al. Transpl Infec Dis 2020;22:e13229.
- Park HY, et al. Chest 2016;150:1222–32.
- Kobayashi T, et al. J Clin Tuberc Other Mycobact Dis 2018;11:17–21.
- Lee MR, et al. PLoS One 2013;8(3):e58214.
- Marras TK, et al. Respir Med 2018;145:80–8.
- Fleshner M, et al. Int J Tuberc Lung Dis 2016;20:582–7.
- Diel R, et al. Eur Resp J 2017;49:1602109.
- Mehta M, et al. Resp Med 2011;105:1718–25.
- Aksamit TR, et al. Chest 2017;151:982–92.
- Andrejak C, et al. Thorax 2013;68:256–62.
- Prevots DR, et al. Clin Chest Med 2015;36:13–34.
- Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
- Axson EL, et al. Eur J Clin Microbiol Infect Dis 2019;38:117–24.
- Park TY, et al. PLoS One 2017;12:e0185774.
- Kotilainen H, et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Wagner D, et al. BMJ Open Respir Res 2020;7:e000498.
- Smith D, et al. BMJ Open Resp Res 2020;7:e000489.
- Griffith DE, et al. Curr Opin Infect Dis 2012;25:218–27.
- Moon SM, et al. Antimicrob Agents Chemother 2016;60:6758–65.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
An overview of the rationale and approach to diagnosis of Mycobacterium avium complex pulmonary disease (MAC-PD)
Mycobacterium avium complex pulmonary disease (MAC-PD) is difficult to diagnose with symptoms similar to underlying lung conditions.1 Correct, early diagnosis and treatment are paramount to prevent disease progression.1–4 The 2020 international guidelines recommend clinical, radiographical and microbiological diagnostic criteria for non-tuberculous mycobacterial pulmonary disease (NTM-PD) to facilitate timely and appropriate treatment.5
NTM-PD: an overlooked disease caused by ubiquitous mycobacteria
Non-tuberculous mycobacteria pulmonary disease (NTM-PD) is a chronic and potentially debilitating disease.6–10 Mycobacteria are ubiquitous in the environment11–13 and comprise almost 200 species and subspecies that can cause opportunistic infections in both pulmonary and extrapulmonary sites.5 NTM infections can be difficult to diagnose and treat and are particularly prevalent in those with lung damage or disease, cancer and immunodeficiencies.14
A series of 12 molecularly related Mycobacterium species have been identified that together comprise the Mycobacterium avium complex (MAC).15 Within MAC, the two most clinically relevant species are M. avium and M. intracellulare.16,17 MAC is the most common cause of NTM.18
Figure 1. Worldwide distribution of respiratory NTM isolates.
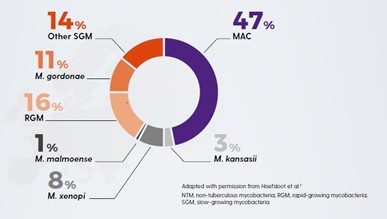
(Adapted from Hoefsloot, 2013)18
Increasing prevalence of NTM-PD
The incidence and prevalence of NTM-PD is increasing in many parts of the world.1,5,19,20 This may reflect increased awareness of the importance of NTM,1 the incidence of risk factors such as chronic obstructive pulmonary disease (COPD) and bronchiectasis,21,22 the use of immunosuppressive treatments,23 testing for NTM-PD and the effectiveness of diagnostic tools.1,17,24 NTM should be ruled out in at-risk patients to identify infection early and begin treatment in appropriate patients before there is disease progression.
Early diagnosis of NTM-PD is paramount to prevent disease progression.
NTM-PD is associated with increased mortality and morbidity – increasing the risk of pulmonary exacerbations9, lung cancer25 and other lung infections26 (e.g. tuberculosis [TB], aspergillosis) and atrial fibrillation.27 NTM-PD is difficult to diagnose as the symptoms of NTM-PD – cough, fatigue, haemoptysis, weight loss – are similar to underlying lung conditions.1 Many patients with NTM-PD may experience symptoms for >10 years before diagnosis.28 Correct, early diagnosis and treatment are paramount to prevent disease progression.1–4 One study has shown that without treatment, 97.5% (n=39/40) of MAC-PD patients will have disease progression within 6 years.17 Another study showed that for those with M. kansasii progression can be very rapid in untreated patients within 1 year (progression in up to 63% of patients) and a median survival of 71 days.29 Late diagnosis, misdiagnosis or inappropriate management of NTM-PD is likely to increase the risk of deterioration in lung health and health-related quality of life.
NTM-PD – not all species are equal
Not all NTM species will cause disease, and when they do, they may not need to be treated.1,30 Likewise the geographical distribution of species differs, driving local epidemiology. Knowledge of the local situation and species virulence is essential for daily clinical practice. MAC is most frequently associated with NTM-PD across all continents with M. avium causing about 63% of infections meeting ATS/IDSA criteria for treatment and M. intracellulare about 88%. Species such as M. gordonae are rarely clinically relevant.30
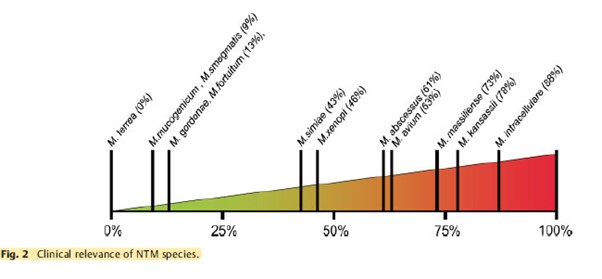
Who is at risk of NTM-PD?
For many at-risk patients NTM-PD symptoms are similar to symptoms of coexisting lung disease.1 These include chronic cough, fatigue, weight loss and low-grade fever.
Patients most at risk of developing NTM-PD include those with:
- Lung diseases e.g. bronchiectasis, COPD, asthma, prior TB.31–34
- Diseases/disorders causing structural lung damage e.g. cystic fibrosis, rheumatoid arthritis, genetic mutation.35–38
- Thoracic skeletal abnormalities – scoliosis, kyphosis, pectus excavatum:39,40 five times more likely to have NTM.34
- Diseases/disorders reducing cell-mediated immunity e.g. HIV AIDS, cancer, genetic mutation.37,38,41
- Therapies resulting in immunodeficiency e.g. organ transplant, anti-tumour necrosis factor (TNF) therapy, corticosteroids, immunosuppressants.34–36,41–44
- Marfanoid body habitus/Lady Windermere syndrome39,40,45 – tall, slender elderly patients with a below normal body mass index (BMI) (<18 Kg/m2) are three times more like to have NTM.34
|
Factors increasing susceptibility to NTM-PD |
Risk* |
|
Bronchiectasis32,47 |
44.0–187.5 |
|
Low BMI47 |
9.1 |
|
Cystic fibrosis65,66 |
6.6–13.0 |
|
COPD47 |
2.0–10.0 |
|
Thoracic skeletal abnormalities47 |
5.4 |
|
Asthma67 |
2.0 |
|
Steroid use47 |
1.6–8.0 |
|
GORD47 |
1.5–5.3 |
|
Immunomodulatory/immunosuppressant therapies47 |
1.3–2.2 |
* Relative risk, odds ratio or relative prevalence
Rates of NTM-PD are high in older individuals and those with underlying bronchiectasis.46,47 Many patients with bronchiectasis also have COPD (36–51%) or asthma (28–42%).23
In which patients should I rule out NTM?
NTM-PD should be ruled out in patients with underlying structural lung disease who:
- are being considered for long-term macrolide therapy to reduce exacerbations48
- are already receiving long-term macrolide therapy48
- present with worsening symptoms despite treatment optimisation49
- present with new pulmonary and non-specific systemic symptoms (e.g. chronic cough, fatigue, fever or dyspnoea).49
MAC-PD: difficult to treat
MAC-PD can be difficult to treat; MAC organisms evade host defences; they accumulate in biofilms and their uptake in macrophages gives them a place to hide from many antibiotics, which have poor penetration of these cells.50–54 Once inside macrophages, MAC limits normal macrophage function and reproduces unhindered, ready to trigger macrophage destruction so MAC bacteria can be released to infect the lung and invade other macrophages.55–57
Diagnostic criteria for MAC-PD
The 2020 international guidelines recommend that MAC-PD is diagnosed with either X-ray or computed tomography (CT) scan and the presence of MAC-positive sputum on multiple occasions. For radiological evidence of MAC-PD, either nodular or cavitary opacities on a chest radiograph, or bronchiectasis with multiple small nodules on a high-resolution CT scan is required. For microbiological confirmation, the 2020 guidelines recommend:5,19
- >1 positive sputum culture (to avoid spurious results from environmental contamination) with >3 respiratory samples collected over 1 week (to distinguish MAC-PD from occasional presence of MAC in the tracheobronchial tract).
- If sputum specimens are not obtainable, bronchoalveolar lavage fluid/bronchial washing cultures can be used to diagnose nodular/bronchiectatic NTM disease.
- Transbronchial or other lung biopsy with mycobacterial histologic features e.g., granulomatous inflammation or acid-fast bacilli (AFB) and positive culture for NTM or biopsy and one or more culture positive sputum or bronchial washings
Species identification helps determine clinical relevance and treatment selection.1,30 Where the same species is isolated in ≥2 sputum cultures over an interval of ≥1 week, there is a 98% likelihood of clinically significant MAC.
Following confirmation of a MAC-PD infection and the decision to treat, the next step is to test for antibiotic susceptibility5 to facilitate the selection of appropriate antimicrobial therapy.
Decision to treat MAC-PD
The decision to initiate antibiotic therapy for MAC-PD should not be based on diagnostic criteria alone.5,19 It is influenced by the severity of the disease, risk of disease progression, species/pathogenicity of infecting Mycobacterium, drug susceptibility, presence of comorbidity and the goals of treatment.1,5,19
Factors favouring treatment include those associated with poor prognosis (e.g. cavitary disease, low BMI, low albumin and elevated inflammatory markers), isolation of a species that is virulent and/or responsive to antimicrobial therapy, underlying immune suppression and major symptoms causing decreased health-related quality of life (e.g. fatigue).5,19 International NTM management guidelines recommend early treatment, as the benefits may outweigh the risks.5 Patients with NTM may already have a high treatment burden from their underlying chronic condition58–60 which could lead to a reluctance to add to it by starting treatment for NTM-PD immediately. However, with evidence from a study which suggests that left untreated, MAC-PD will progress,17 and increase risk of all-cause mortality27,61 and morbidity6–9,62 lowering patient quality of life,26,63 prompt treatment is essential.
Summary
Understanding which patients with underlying lung conditions are at risk of developing MAC-PD is important in order to deliver prompt and effective therapy. Treatment for MAC-PD is most successful at first initiation1 and a study has shown that refractory disease is associated with use of non-standard treatment regimens as outlined in the guidelines.64
Understanding the need to identify at-risk patients, implement effective diagnostic protocols and prompt robust therapy is a medical imperative with the potential to reduce patient mortality and morbidity.
References:
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Eikhani MS, et al. BMC Infect Dis 2018;18:311.
- Maiga M, et al. PLoS One 2012;7:e36902.
- Wagner D, et al. Poster presented at: European Respiratory Society Annual Congress 6–10 September 2014; Munich, Germany. P1067.
- Daley CL, et al. Eur Respir J 2020b;56(1):2000535.
- Park HY, et al. Chest 2016;150:1222–32.
- Kobayashi T, et al. J Clin Tuberc Other Mycobact Dis 2018;11:17–21.
- Lee MR, et al. PLoS One 2013;8:e58214.
- Huang CT, et al. Int J Tuberc Lung Dis 2012;16:539–45.
- Marras TK, et al. Emerg Infect Dis 2017;23:468–76.
- Falkinham JO. J Appl Microbiol 2009;107:356–671.
- Falkinham JO. Clin Chest Med 2015;36:35–41.
- Nishiuchi Y, et al. Front Med 2017;4:27.
- Ratnatunga CN, et al. Front Immunol 2020;11:303.
- van Ingen J, et al. Int J Syst Evol Microbiol 2018;68:3666–77.
- Boyle DP, et al. Am J Respir Crit Care Med 2015;191:1310–17.
- Park TY, et al. PLoS One 2017;12:e0185774.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Daley CL, et al. Clin Infect Dis 2020a;71:e1–e36.
- Diel R, et al. BMC Infect Dis 2018;18:206.
- Terzikhan N, et al. Eur J Epidemiol 2016;31:785–92.
- Snell N, et al. Respir Med 2019;158:212–3.
- Chalmers JD, et al. Pulmonol 2018;24:120–31.
- Chalmers JD, et al. Chest 2018;165:1272–3.
- Taira N, et al. Am J Case Rep 2018;19:748–51.
- Yeung MY, et al. Respirology 2016;21:1015–25.
- Park CS, et al. Sci Rep 2019;9:15503.
- Kotilainen H, et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
- Liu C-J, et al. Respir Med 2019;151:19–26.
- Zweijpfenning SM, et al. Semin Respir Crit Care Med 2018;39:336–42.
- Aksamit TR, et al. Chest 2017;151:982–92.
- Andrejak C, et al. Thorax 2013;68:256–62.
- Jones MM, et al. PLoS One 2018;13:0197976.
- Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
- Winthrop KL, et al. Ann Rheum Dis 2013;72:37–42.
- Brode SK, et al. Thorax 2015;70:677–82.
- Wu UI, et al. Lancet Infect Dis 2015;15:968–80.
- Szymanski EP, et al. Am J Respir Crit Care Med 2015;192:618–28.
- Kim RD, et al. Am J Respir Crit Care Med 2008;178:1066–74.
- Holt MR, et al. Eur Respir J 2019;54:1900252.
- Henkle E, et al. Clin Chest Med 2015;36:91–9.
- Ose N, et al. Surg Case Rep 2019;5:11.
- Friedman DZP, et al. Transpl Infect Dis 2020;22:e13229.
- Chao WC, et al. BMC Infect Dis 2017;17:796.
- Ku JH, et al. Diagn Microbiol Infect Dis 2020;96:114916.
- Prevots DR, et al. Am J Respir Crit Care Med 2010;182:970–6.
- Prevots DR, et al. Clin Chest Med 2015;36:13–34.
- Smith D, et al. BMJ Open 2020;7:e000489.
- Haworth C, et al. Thorax 2017;72:ii1–ii64.
- Awuh JA, et al. Cell Mol Life Sci 2017;74:1625–48.
- Ganbat D, et al. BMC Pulm Med 2016;16:19.
- Esteban J, et al. Front Microbiol 2018;8:2651.
- Chakraborty P, et al. Microbiol Cell 2019;6:105–22.
- McGarvey, et al. Clin Chest Med 2002;23:569–83.
- Chiplunkar SS, et al. Future Microbiol 2019;14:293–313.
- Gomes MS, et al. Infect Immun 1999;67:3199–206.
- Lee K-I, et al. Sci Rep 2016;6:37804.
- Global Burden of Disease 2015 Chronic Respiratory Disease Collaborators. Lancet Respir Med 2017;5:691–706.
- Lopez-Campos J, et al. Respirology 2016;21:14–23.
- Redondo M, et al. Breathe 2016;12:222–35.
- Diel R, et al. Eur Resp J 2017;49:1602109.
- Fleshner M, et al. Int J Tuberc Lung Dis 2016;20:582–7.
- Mehta M, et al. Respir Med 2011;105:1718–25.
- Fukushima K, et al. J Clin Med 2020;9:1315.
- Olivier KN, et al. Am J Respir Crit Care Med 2003;167:828–34.
- Roux A-L, et al. J Clin Microbiol 2009;47:4124–8.
- Hojo M, et al. Respirology 2012;17:185–90.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Understanding best practice in Mycobacterium avium complex pulmonary disease (MAC-PD)
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) varies depending on the species, extent of disease, drug susceptibility results and underlying comorbidities.1 Mycobacterium avium complex (MAC) is the most common cause of disease and a multidrug antimicrobial regimen is recommended, first line, to avoid the development of resistance.1,2 Treatment is lengthy and adverse events are not uncommon; successful treatment requires good adherence, frequent monitoring and effective adverse event management.1,3
Initiation of treatment for MAC-PD
Treatment of MAC-PD with antimicrobial agents offers the possibility of cure of the disease.1 In patients who meet the diagnostic criteria (clinical symptoms, radiological evidence of nodules, bronchiectasis or cavitary opacity, and microbiological evidence of positive culture results from sputum), the ATS/ERS/ESCMID/IDSA 2020 NTM guidelines suggest initiation of treatment rather than watchful waiting, especially in the context of positive acid-fast bacilli sputum smears and/or cavitary lung disease.1 The decision to initiate antimicrobial therapy should nonetheless be individualised and based on a combination of clinical factors, the infecting species and individual patient priorities.1,4
Comprehensive care and good communication across a multidisciplinary team of healthcare providers is important because of the complexities of MAC-PD management and treatment.5–7 When initiating treatment, setting expectations for the patient is critical and it is important to discuss length of therapy, treatment response, follow-up appointments and potential adverse events.1
“One needs to discuss with the patient the likely side-effects of treatment, the likely success or failure of treatment and, of course, the potential for reinfection.” Charles Haworth, Royal Papworth Hospital, UK
“If you need to start treatment that you’re inexperienced with or you run into toxicity or other issues that you’re inexperienced in, consult centres of excellence in this field to improve management of the patient.” Jakko van Ingen, Radboud UMC, the Netherlands
Drug susceptibility testing for MAC-PD
Macrolide monotherapy is commonly prescribed in NTM-PD and can be a key driver in the development of macrolide-resistant strains, leading to poor outcomes for NTM treatment.8
Given the good correlation between in vitro activity and in vivo outcomes with macrolides9 and amikacin3 for MAC, the 2020 NTM guidelines recommend susceptibility-based treatment for macrolides and amikacin over empirical therapy for patients with MAC-PD.1 Recommendations, including protocols and related quality-control parameters, for the susceptibility testing of mycobacteria are provided by the Clinical and Laboratory Standards Institute (CLSI).10
“Drug susceptibility testing should be performed for the macrolides and amikacin before the onset of treatment.” Jakko van Ingen, Radboud UMC, the Netherlands
“For patients with MAC lung disease, it’s essential to perform drug susceptibility testing on isolates before you commence treatment. In particular, it’s crucial to know the macrolide susceptibility as that will influence the initial regimen and it’s also important to know the amikacin susceptibility, particularly in patients with severe disease or cavitary disease.” Charles Haworth, Royal Papworth Hospital, UK
Multidrug treatment regimen for MAC-PD
Patients respond best to MAC treatment regimens the first time they are administered; therefore, it is especially important that patients receive recommended multidrug therapy the first time they are treated for MAC-PD, especially as up to 45% of patients are known to fail first-line therapy.11–14
Macrolides (clarithromycin and azithromycin) are a key component of MAC-PD treatment based on data that shows poor patient outcomes if they are excluded.1 The 2020 NTM guidelines recommend treatment with at least three antimicrobials (Table 1) for macrolide-sensitive MAC-PD with the addition of parenteral amikacin or streptomycin in macrolide-insensitive disease or advanced bronchiectatic/cavitary disease.1
Macrolide resistance is associated with higher mortality rates than macrolide-sensitive disease, with one study suggesting a mortality rate of >45% over 5 years.8 Treating macrolide-resistant disease can be difficult and expert consultation should be sought.1
“The most important thing in treating macrolide-resistant MAC pulmonary disease is to look for help… seek expert consultation.” Jakko van Ingen, Radboud UMC, the Netherlands
Table 1. Guideline-based therapy for Mycobacterium avium complex pulmonary disease (MAC-PD).1
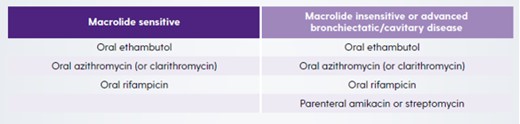
For both macrolide-sensitive and macrolide-insensitive disease, azithromycin is recommended over clarithromycin because of better tolerance, fewer drug interactions, lower pill burden and equal efficacy.1 However, when azithromycin is not available or not tolerated, clarithromycin is considered an acceptable alternative.1 Ethambutol is included in the recommendation as it is the most effective drug known to prevent the development of macrolide resistance.15,16
“The three-drug regimen should be based on azithromycin as the macrolide of choice, plus ethambutol as a second drug and a third companion drug, most likely being a rifamycin. In the case of severe disease, like fibro-cavitary disease or disease that affects both lungs, additional treatment with a parenteral aminoglycoside like streptomycin or amikacin should be considered.” Christoph Lange, Research Center Borstel, Germany
“The guidelines are very clear that azithromycin is preferable over clarithromycin in most circumstances and that’s because, on the whole, it’s better tolerated particularly from a gastrointestinal perspective, it’s once a day rather than twice a day and there are fewer drug–drug interactions, particularly with rifampicin.” Charles Haworth, Royal Papworth Hospital, UK
In patients with non-cavitary nodular/bronchiectatic disease a dosing regimen of three times per week is recommended but in patients with cavitary severe/advanced disease, treatment should be administered daily.1
Monitoring for treatment response in MAC-PD
The 2020 NTM guidelines recommend frequent follow-up visits after initiating treatment for MAC-PD, including obtaining sputum cultures every 1 to 2 months to determine if, and when, culture conversion occurs.1 Retrospective studies have shown that among the NTM-PD patients who convert on standard first-line multidrug treatment, the majority do so within 6 months of treatment initiation.17–19 In addition to microbiological assessments, clinical and radiographical findings should also be used to determine if the patient is responding to therapy.1
“One of the major mistakes done in the management of patients with Mycobacterium avium complex pulmonary disease or any other NTM pulmonary disease, is the lack of proper monitoring, for example monthly sputum collection for microscopy and culture.” Christoph Lange, Research Center Borstel, Germany
If patients do not respond as expected, therapeutic drug monitoring could be considered in situations where drug malabsorption, drug underdosing or clinically important drug–drug interactions are suspected.20
It is recommended that treatment should be maintained for at least 12 months after culture conversion to increase chances of treatment success.1 Of note, in a study of 154 patients with MAC-PD, those who were treated for <15 months after culture conversion were twice as likely to experience recurrence than those treated for ≥15 months post conversion.17
Management of patients with MAC-PD who fail to culture convert
Study data has shown that up to 45% of MAC-PD patients will fail to respond on standard first-line multidrug treatment.13,14 Non-conversion may be an early sign that the patient may have future radiographic progression and lung function decline.21,22
In cases of MAC-PD where sputum cultures do not convert after 6 months of treatment, the 2020 NTM guidelines recommend that Amikacin Liposomal Inhalation Suspension (ALIS) once daily is added to the regimen.1
“When mycobacterial cultures do not convert by 6 months on oral guideline-based therapy patients should be considered to receive amikacin liposomal inhalation suspension. This has been shown to increase the chances to achieve culture conversion by 12 months and the effect of culture conversion is sustained for at least 3 months past the end of therapy.” Christoph Lange, Research Center Borstel, Germany
In selected patients with failure of medical management, cavitary disease, drug-resistant isolates or complications such as severe bronchiectasis, surgical resection of the diseased lung may be appropriate. The risks and benefits of surgery should be weighed up and expert consultation sought.1
“Surgical resection of the most affected areas of the lung can help to achieve cure in patients.” Jakko van Ingen, Radboud UMC, the Netherlands
“In patients with macrolide-resistant MAC-PD, we should also consider the role of surgery. These patients should be discussed with an expert surgeon because in some cases surgery could be on top of treatment and could improve outcomes for patients with macrolide-resistant MAC-PD.” Stefano Aliberti, University of Milan, Italy
Monitoring for adverse reactions in treatment of MAC-PD
The drugs routinely used to treat MAC-PD are frequently associated with adverse reactions,1 as demonstrated in a recent randomised clinical trial where >90% of participants experienced an adverse event.3 Consequently, educating patients regarding potential adverse reactions and monitoring them are important components of patient management. Furthermore, rapid identification and management of an adverse reaction may decrease the risk of treatment discontinuation and possibly improve the chances of treatment completion.1 Where drug intolerance is suspected, some medications could be introduced gradually at 1- to 2-week intervals so that appropriate evaluations of tolerance can be performed.11 According to the ATS/ERS/ESCMID/IDSA 2020 guidelines, it is important to individualise the frequency of monitoring for adverse reactions based on patient age, comorbidities, concurrent drugs, overlapping drug toxicities and resources.1 It is recommended that patients should have a complete blood count, liver function tests and metabolic panel every 1–3 months in patients on oral therapy and weekly if on intravenous therapy.1 Depending on the antibiotics selected, there may be a need to refer to other specialists for routine monitoring, including an ophthalmologist for vision testing and an audiologist to take baseline audiograms and hearing tests (Table 2).1
“Blood monitoring is essential because many of the treatments are quite toxic, so you’ll do full blood counts to look for bone marrow toxicity, liver blood tests and renal monitoring…Depending on the regimen you may want to do an ECG, to check for QT prolongation and often we’ll do audiology in patients that are on azithromycin or amikacin, to look for evidence of hearing impairment.” Charles Haworth, Royal Papworth Hospital, UK
Table 2. Common adverse reactions associated with drugs used to treat Mycobacterium avium complex pulmonary disease (MAC-PD) and monitoring recommendations.1
|
Drug |
Adverse Reactions |
Monitoring |
|
Azithromycin/clarithromycin |
Gastrointestinal Tinnitus/hearing loss Hepatotoxicity Prolonged QTc |
Clinical monitoring Audiogram Liver function tests ECG (QTc) |
|
Ethambutol |
Ocular toxicity
Neuropathy |
Visual acuity and colour discrimination Clinical monitoring |
|
Rifampicin |
Hepatotoxicity Cytopenias Hypersensitivity Orange discoloration of secretions |
Liver function test Complete blood count Clinical monitoring |
|
IV Amikacin/Streptomycin |
Vestibular toxicity Ototoxicity Nephrotoxicity Electrolyte disturbances |
Clinical monitoring Audiograms BUN, creatinine Calcium, magnesium, potassium |
BUN, blood, urea, nitrogen; ECG, electrocardiogram.
Best practice beyond pharmacotherapy alone
Providing best practice in MAC-PD requires an holistic approach to patients that explores non-pharmacological interventions as well as medication regimens outlined in the ATS/ERS/ESCMID/IDSA 2020 guidelines.
From the point of diagnosis, patients should be encouraged to undertake airway clearance techniques in order to limit lung function decline.23 The patient journey for MAC-PD patients is long, and many will have lived with their condition for some time before diagnosis.24 Living with a chronic, potentially debilitating disease is hard for patients and has a negative impact on their quality of live. Psychological interventions may be relevant for these patients and should be considered. Similarly, it is known that low body mass index is a risk factor for NTM-PD and is often a clinical characteristic of these patients. In these patients, nutritional support is important and involving a nutritionist or dietician in the clinical team may be helpful as improving diet and stopping weight loss can help to counter low mood.23
Summary
Patients with MAC-PD often face a long and punishing treatment journey with lengthy and complex multidrug regimens, many of which are associated with adverse events that affect patient adherence. Best practice management for MAC-PD should consider all aspects of the patient’s journey from non-pharmacological interventions, such as airway clearance, through to collaboration with other specialists for example radiologists, respiratory physicians, psychologists, dieticians, specialist pharmacists, nurses and physiotherapists to optimise patient’s nutrition, sleep and mental state.
References:
- Daley CL, et al. Eur Respir J 2020;56(1):2000535.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Griffith DE, et al. Am J Respir Crit Care Med 2018;198:1559–69.
- Haworth C, et al. Thorax 2017;72:iii1-ii64.
- Ryu YJ, et al. Tuberc Respir Dis (Seoul) 2016;79:74–84.
- Yu JA, et al. Thorac Surg Clin 2012;22:277–85.
- van Ingen J. Semin Respir Crit Care Med 2013;34:103–9.
- Moon SM, et al. Antimicrob Agents Chemother 2016;60:6758–65.
- Kobashi Y, et al. J Infect Chemother 2006;12:195–202.
- Clinical and Laboratory Standards Institute. M48 - laboratory detection and identification of mycobacteria, 2nd edition 2018Clinical and Laboratory Standards Institute. M24 – Susceptibility Testing of Mycobacteria, Nocardia spp, and other Aerobic Actinomyces, 3rd edn. 2018.
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Griffith DE, Aksamit TR. Curr Opin Infect Dis 2012;25:218–27.
- Fukushima K, et al. J Clin Med 2020;9:1315.
- Wallace RJ, et al. Chest 2014;146:276–82.
- Griffith DE, et al. Am J Respir Crit Care Med 2006;174:928–34.
- Morimoto K, et al. Ann Am Thorac Soc 2016;13:1904–11.
- Furuuchi K, et al. Chest 2020;157:1442–5.
- Koh WJ, et al. Eur Respir J 2017;50:1602503.
- Moon SM, et al. Eur Respir J 2019;53:1801636.
- Nahid P, et al. Clin Infect Dis 2016;63:e147–95.
- Park HY, et al. Chest 2016;150:1222–32.
- Pan SW, et al. Clin Infect Dis 2017;65:927–34.
- Lipman M, et al. BMJ Open Resp Res 2020;7:e000591.
- Kotilainen H, et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Understanding the risk factors that underlie NTM-PD
The ERS/ATS/ESCMID/IDSA 2020 NTM-PD guideline gives an overview of how to diagnose a patient for NTM-PD, relying on clinical, radiological and microbiological evaluations.1 But when a patient walks into the clinic, what is it about that individual that might prompt a clinician to consider testing for NTM?
Understanding the risk factors that are common in patients with NTM-PD provides a valuable insight into patients who might benefit from testing for NTM to rule out disease and, if NTM infection is present, what might be the appropriate course of action. Risk factors for NTM-PD include those inherent to the patient themselves (host risk factors), environmental risk factors (exposure), immunological risk factors, genetic risk factors and the presence of underlying lung conditions or disease.2
|
Factors increasing susceptibility of NTM-PDFactors |
Increased risk or prevalence of infection* |
|
Bronchiectasis |
44–187.53,4 |
|
Previous TB |
69.0–178.34,5 |
|
Low BMI |
9.13 |
|
Cystic fibrosis |
6.6–13.06,7 |
|
COPD (in receipt of ICS) |
29.14 |
|
COPD (no ICS) |
2.0–10.03 |
|
Thoracic skeletal abnormalities |
5.43 |
|
Lung cancer |
3.43 |
|
Asthma |
2.0–7.84,8 |
|
Steroid use |
1.6–8.03 |
|
GERD |
1.5–5.33 |
|
Rheumatoid arthritis |
1.5–1.93 |
|
Immunomodulatory/immunosuppressant therapy |
1.3–2.23 |
*Odds ratio, relative risk or relative prevalence.
Factors which increase risk of NTM-PD should be considered when deciding which patients to screen or test for infection.
Underlying lung conditions
Bronchiectasis
Bronchiectasis is known to be a risk factor for NTM-PD, but the scale of risk is variably estimated.4,9,10 NTM infection can cause bronchiectasis, and in patients with existing bronchiectasis can facilitate disease progression as the anatomically altered bronchi are susceptible to infection.11 The increased risk for developing NTM-PD in patients with bronchiectasis is estimated between 44 and 187.5-fold3,4 and the prevalence of NTM-PD among people with bronchiectasis was suggested to be 9.3%.12
Bronchiectasis is associated with NTM-PD in patients who are female and have a low-fat mass even after adjustments for BMI, age and fat mass index.13 A study in the USA has attempted to predict which patients with bronchiectasis might also have NTM-PD.14 In this study results indicated that >2 claims to a healthcare insurer within 12 months and 30 days apart of each other accurately identified pulmonary NTM infection in patients who also have bronchiectasis. However, the authors noted that the prediction had low sensitivity so that the true incidence might be severely underestimated. Importantly, a recent study11 demonstrated that NTM-PD in patients with bronchiectasis was associated with radiological changes and worsening symptoms yet despite this the Bronchiectasis Severity Score (BSI) may remain unchanged. Consequently, it is recommended that patients with bronchiectasis are tested for NTM organisms in sputum, particularly those with radiographic changes. Similarly, the ERS and British Thoracic Society (BTS) guidelines15,16 recommend that all patients with bronchiectasis being considered or receiving macrolide monotherapy should have NTM-PD ruled out.
People with bronchiectasis have an increased risk of developing NTM-PD between 44 and 187.5 fold vs those without bronchiectasis3,4
Cystic fibrosis
NTM-PD is increasing among cystic fibrosis (CF) populations worldwide. The reasons for this are unclear but may reflect the increasing lifespan of patients and the greater success that has been achieved in controlling bacterial infections like Pseudomonas aeruginosa, as well as greater awareness leading to increased NTM-PD diagnoses.7,17 Current estimates outlined in one study are that NTM infects up to 32% of CF.18 Risk of NTM-PD in people with cystic fibrosis is high with an increased prevalence of infection ranging from 6.6 to 13-fold.6,7 (Olivier 2003; Roux 2009).
A meta-analysis has demonstrated that people with CF were significantly more likely to be positive for NTM cultures if they were older (p<0.01), had Aspergillus fumigatus colonisation (OR 3.59 p<0.001), were colonised with Staphylococcus aureus (OR 1.66 p=0.001) or Stenotrophomonas maltophilia (OR 3.41 p<0.01) or were in receipt of ICS (OR 1.98 p<0.01); no other parameter showed a significant association.19 Taken together, patients with CF who also have severe conditions or infections should be closely monitored for NTM-PD.
In CF the risk of developing NTM-PD in increased, with the rates of NTM-PD in patients with CF up to 13-fold higher than in the general population,6 particularly in older patients and those with bacterial colonisation or in receipt of ICS19
COPD
COPD is a common comorbidity with NTM-PD, and a predictive modelling study20 showed that COPD was one of the highest single predictors for NTM-PD. In the USA, rates of NTM-PD in patients with COPD have been increasing since 2012,21 more than doubling between 2011 and 2015 (compared with an increase of a quarter between 2001 and 2005). Infection with NTM in patients with COPD elicited an increased mortality risk of 1.43 times that of patients with COPD and no NTM-PD,21 and a Canadian study indicated an OR for COPD as a risk factor of 15.7.4
A smaller study demonstrated that in patients with COPD, once BMI, forced expiratory volume (FEV1), and ICS use are discounted rates of NTM-PD are higher than in the general population.22 Similarly, the hazard ratio for NTM-PD in patients with pre-existing COPD was 9.15 when adjusted for sex and age, reducing only to 6.01 when fully adjusted for all factors.23 These data are important as the Canadian study by Marras et al. was a population-based study on >6 million people and the association with NTM-PD underlying COPD was studied in detail.
The overall increasing rates of COPD and the risk of NTM-PD in COPD patients indicates that changes in symptoms or radiographic changes should be investigated with NTM infection front of mind.
When other risk factors such as BMI, lung function and ICS use are discounted, COPD remains a risk factor for increased infection with NTM – up to 15.7 fold3,22,23
Asthma
Patients in receipt of inhaled corticosteroids (ICS) are known to be at increased risk of NTM infection.4,24 In one study the increase in risk of NTM-PD associated with asthma per se was 7.8.4 A nested case-control study specifically explored the association between asthma and NTM infection and suggested that patients with asthma were older, had severe airflow restriction and had been in receipt of ICS for longer (>5 years) at higher doses.8 The authors did note that receipt of ICS in these patients may be a contributing risk factor. Taken together it seems rational that asthma with its characteristic inflamed and constricted airways provides an independent risk factor for NTM-PD that is likely overlaid by additional risk because of ICS use.
Asthma increases the risk of NTM-PD up to 7.8 fold4
Immunosuppressed patients
It has long been known that using ICS increases the risk of pneumonia.25 A study in patients with asthma, COPD or COPD-asthma syndrome aged >66 years were evaluated to compare ICS use or not in those with NTM-PD. Current ICS use was associated with an increased OR of NTM-PD of 1.86 and was statistically significant for fluticasone (OR 2.09) over budesonide (OR 1.19) and the relationship between disease and ICS use was dose-dependent.24
In rheumatoid arthritis (RA) the use of biologics such as anti-tumour necrosis factor (anti-TNF) is associated with an increased risk of developing NTM-PD26 and were highest for those in receipt of adalimumab over infliximab and etanercept: 5–10 fold higher than those in RA patients not exposed to anti-TNF therapy or the general population.26,27 Similarly, in South Korea patients treated with anti-TNF a higher incidence of NTM-PD of 230 cases per 100,000 patients was reported,27 and in the USA and South Korea 70–100% of patients with RA and underlying lung disease had NTM-PD, suggesting a cumulation of risk.27 estimates the increased risk for NTM-PD in patients in receipt of immunomodulatory or immunosuppressant therapy overall at 1.3 to 2.2-fold.3
Other biologics such as rituximab (used for cancer and RA among others), abatacept, tocilizumab and ustekinumab have a theoretical increased risk of NTM-PD, but at the current time no studies are available and data is limited to small case series only.27 Similarly, in patients in receipt of immunosuppressants following transplant such as tacrolimus, NTM-PD has been identified, but again the data are limited to small case series or individual case reports.28,29
Whilst rarely seen now, an increase in NTM-PD was primarily identified in people with HIV/AIDS because of their immunosuppressed state.27 With the advent of highly active anti-retroviral therapy (HAART) there has been a sharp decline in NTM-PD cases, and it is now uncommon in people living with HIV.
For patients in receipt of immunosuppressants, particularly those with underlying lung disease, there is a need to screen those where clinical suspicion is raised. It is also vital that clinical colleagues in rheumatology or transplant medicine understand the theoretical risk for immunosuppressive therapy and NTM-PD so they can refer appropriately and promptly.
Overall, the increase in risk for NTM-PD as a result of receiving immunomodulatory or immunosuppressant therapy is 1.5–2.2 fold3
Host risk factors: patient morphology
Patient characteristics that predispose individuals to NTM-PD are well known and include:30
- Biological sex – females are at greater risk
- Thoracic skeletal abnormalities including pectus excavatum and scoliosis
- Taller than average (>165 cm for women)
- Low BMI (<20 Kg/m2)
- Leaner skinfold and limb circumference measurements than those without NTM infection.
These morphological characteristics have been initially described as those of Lady Windermere, after the character in the Oscar Wilde play Lady Windermere’s Fan,31 but it must be noted that similar morphological characteristics in males are also associated with an increased risk of NTM-PD, called Lord Windermere syndrome.32
Patient morphology can also influence disease progression once NTM-PD is diagnosed, with those with low proportions of abdominal fat at increased risk of disease progression.33 One case-controlled study indicated that tall height and a thoracic abnormality was associated with an increased odds ratio (OR) for NTM-PD of 1.1, and 5.4, respectively, whilst a BMI above normal (>26 kg/m2) was shown to be protective for NTM-PD (OR 0.11).34 Similarly, a low BMI was associated with an increased risk of NTM-PD of 9.1 fold.3 Family studies have suggested clustering of NTM-PD among related family patients with at-risk morphological characteristics suggesting an underlying genetic component.3,35
Morphological characteristics confer an increased risk of NTM-PD, tall height increases risk by 1.1-fold, thoracic abnormalities 5.4 fold and low BMI 9.1 fold3,34
Genetic predisposition
A range of genetic abnormalities are associated with an increased risk of NTM-PD and these include CF as outlined above, α1-antitrypsin deficiency (AATD) and primary ciliary dyskinesia (PCD). In both AATD and PCD, predisposition to NTM-PD is due to their impact on the lung. For AATD the appearance of COPD is very common after 30 years of age.36 In PCD genetic mutations lead to dysfunctional and uncoordinated cilial function in the lung leading to bronchiectasis; the prevalence of NTM-PD in PCD is estimated to be about 15%.37 In patients with PCD screening for NTM-PD should be done in the same way as for CF and regular sputum culture is recommended every 3–6 months.37 Similarly, in both CF and PCD training to undertake effective airway clearance is essential for patients to clear the lung to prevent infection and, if NTM-PD is present, to prevent lung damage.
Primary immune deficiency syndromes such as the rare condition Mendelian Susceptibility to Mycobacterial Disease (MSMD) is a risk factor for NTM-PD. MSMD is associated with interleukin receptor abnormalities and deformities in genes in the inflammatory cascade process.38 Likewise, a host of genetic abnormalities in the inflammatory pathway including gamma interferon (IFNγ) interleukins (IL-12) and IFN/IL receptors and macrophage proteins such as natural-resistance-associated macrophage protein 1 gene (NRAMP1) are known risk factors for NTM-PD,39 perhaps suggesting an inflammatory component to disease.
Previous or current TB
In a recent study,40 an evaluation of NTM-PD in TB suggested that one in 15 patients with TB in China also have NTM infection. The most common infecting species were M. intracellulare and M. abscessus complex. A similar co-infection state was demonstrated some years ago, with the authors suggesting that TB infection for the majority of patients follows within 6 months of confirmed NTM-PD and is significantly more common in people with a previous history of NTM-PD.41 Conversely previous TB as a pre-disposing factor to NTM-PD was suggested to confer an OR of 69.0 in the UK whilst in Denmark the risk conferred was more than doubled with an OR of 178.3.4,5
Whilst TB may be on the decline in Europe,42 it continues to be endemic in some countries, and the rise of rifampicin and multi-drug resistant TB in Europe means it remains an important disease consideration. When a patient with symptoms of NTM-PD presents and the suspicion of infection arises, patients should always be screened for previous TB.
Gastroesophageal reflux disease (GERD)
Other pre-disposing risk factors include GERD42 with an estimated increased risk of 1.5–5.3 fold.3 Patients with GERD are more likely to have acid-fast NTM bacilli and NTM-PD compared with patients without GERD and similarly, patients with MAC-PD show a high rate of GERD as a co-morbidity.42,43 The prevalence of GERD in patients with nodular NTM-PD is about 26%43 even when adjusted for other factors such as age, sex, BMI and lung function tests. Patients with GERD and NTM-PD who are in receipt of acid supressing medications are more likely to have consolidation and nodules of a size greater than 5 mm.42 It is likely that GERD is a risk factor as a result of aspiration of gut bacteria into the lung, and it is thought that acid suppression supports bacterial proliferation and survival in the gut.42
Environmental exposure
NTM are ubiquitous in the environment in soil and water supplies,43–45 and it has been considered that the environment is the reservoir for human infection. Increased NTM-PD prevalence has been linked to increased average atmospheric water vapour content,46 and the presence of NTM in home water supply is more common in patients with NTM-PD than those without disease,47 increasing the risk of NTM-PD up to 5.9-fold.3 NTM are also present in a range of other environmental and domestic scenarios but their role in infection is unclear.2 Given the pervasive nature of NTM mitigating infection is difficult, but in patients at risk simple steps such as replacing water filters regularly, avoiding hot tubs, cleaning showerheads regularly and wearing gloves when gardening can be helpful.
In summary
It is clear from a growing body of research that there exists a raft of potential risk factors that would lead a clinical suspicion of NTM-PD. But it is known that not every tall, slender woman or every patient with COPD, whilst at increased risk of NTM-PD, will develop disease, and so refining clinical suspicion is essential. These include consideration of changes or worsening in clinical condition of the patient at high risk of NTM-PD, an understanding of their medical and social history to understand iatrogenic risks in a patient’s life, and then to test and monitor patients where NTM-PD is suspected is an imperative.
Test and monitor patients with potential clinical symptoms or worsening clinical symptoms who fit the NTM-PD profile:
- Tall, slender men or women
- Patients with underlying lung conditions: asthma, bronchiectasis, COPD
- Patients with genetic conditions: CF, AATD, PCD
- Patients with GERD or environmental exposure
What to do? Rule out the presence of NTM in patients with risk factors to identify early infection and follow current treatment guidelines1 to start treatment to prevent disease progression and achieve culture conversion. Think NTM! Test NTM!
References:
1. Daley CL, et al. Eur Respir J 2020;56:2000535.
2. Cowman S, et al. Eur Respir J 2019;54:1900250.
3. Prevots DR, et al. Clin Chest Med 2015;36:13–34.
4. Andrejak C, et al. Thorax 2013;68:256–62.
5. Axson EL, et al. Eur J Clin Microbiol Infect Dis 2019;38:117–24.
6. Olivier KN et al. Am J Respir Crit Care Med 2003;167:828-34
7. Roux A-L, et al. J Clin Microbiol 2009;47:4124–28.
8. Hojo M, et al. Respirology 2012;17:185–90.
9. Shteinberg M, et al. Eur Respir J 2018; 51:1702469.
10. Ringshausen FC, et al. Emerg Infect Dis 2016;22:1102–05.
11. Chu H, et al. Arch Med Sci 2014;10:661–68.
12. Lim SY, et al. Medicine (Baltimore) 2021;100:e25193.
13. Ku JH, Diagn Microbiol Infect Dis 2020;96:114916.
14. Kwak N, et al. BMC Pulm Med 2020;20:293.
15. Smith D, et al. Thorax 2020;0:1–35.
16. Polverino E, et al. Eur Resp J 2017 ;50 :1700629.
17. Salsgiver EL, et al. Chest 2016;149:390–400.
18. Floto RA, et al. Thorax 2016;71:88–90.
19. Reynaud Q, et al. Pediatr Pulm 2020;55:2653–61.
20. Ringshausen FC, et al. Int J Infect Dis 2021;104:398–406.
21. Pyarali FF, et al. Front Med 2018;5:311.
22. Okamuri S, et al. Eur Respir J 2015;46:PA569.
23. Marras TK, et al. Eur Respir J 2016;48:928–31.
24. Brode SK, et al. Eur Respir J 2017;50:1700037.
25. Suissa S, et al. Thorax 2013;68:1029–36.
26. Winthrop KL, et al. Ann Rheum Dis 2013;72:37–42.
27. Henkle E, et al. Clin Chest Med 2015;36:91–99.
28. Suzuki H, et al. Transplant Proc 2018;50:2764–67.
29. Imoto W, et al. BMC Infect Dis 2020;20:431.
30. Kim RD, et al. Am J Respir Crit Care Med 2008;178:1066–74.
31. Reich JM, et al. Chest 1992;101:1605–09.
32. Ku JH, et al. Emerg Infect Dis 2021;27:982–85.
33. Kim SJ, et al. BMC Pulm Med 2017;17:5.
34. Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
35. Colombo RE, et al. Chest 2010;137:629–34.
36. Stoller JK, et al. 2006 Oct 27 [Updated 2020 May 21]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021. https://www.ncbi.nlm.nih.gov/books/
37. Daniels MLA, et al. Exp Opin Orphan Drugs 2015;3:31–44.
38. Ratnatunga CN, et al. Front Immunol 2020;11:303.
39. Baldwin SL, et al. PLOS Neglected Trop Dis 2019;13:e0007083.
40. Tan Y, et al. J Infect 2021;S0163-4453(21)00261-9.
41. Hsing S-C, et al. Int J Tuberc Lung Dis 2013;17:928–33.
42. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2019 Accessed July 2021
42. Thomson RM et al. Chest 2007;131:1166-72
43. Koh W-J, et al. Chest 2007;131:1825–30.
43. Lee E-S, et al. J Microbiol Biotechnol 2008;18:1207–15.
44. Falkinham JO. Clin chest Med 2015;36:35–41.
45. Falkinham JO. J Appl Microbiol 2009;107:356–67.
46. Prevots DR, et al. Annals Am Thorac Soc 2014;11:1032–38.
47. Nishiuchi Y, et al. Clin Infect Dis 2007;45:347–51.
NTM-PD at the European Respiratory Society congress 2021
Insmed-sponsored symposium ‘Innovations in MAC-PD: beyond oral triple therapy’
In this symposium, the faculty of Professor Christoph Lange, Professor Stefano Aliberti and Dr Natalie Lorent, chaired by Professor Michael Loebinger, provided an overview of the recently updated MAC-PD guidelines as a tool to identify and treat patients, a summary of new recommendations and the management of adverse events (AEs) commonly associated with MAC-PD treatment.
Professor Lange presented the clinical relevance of NTM infection and the pathogenicity of NTM species, with M. avium, M. intracellulare, M. kansasii, M. xenopi, and M. abscessus being most important in NTM-PD.1 He demonstrated how bronchiectasis and MAC-PD are inter-related with respect to inflammation and lung damage that can drive a high mortality rate and a relatively poor rate for culture conversion during treatment,2–4 which is even worse for patients with refractory MAC-PD.5 He discussed the role of parenteral aminoglycosides in severe MAC-PD that, whilst effective against MAC-PD, can increase specific side effects including vestibular toxicity, ototoxicity and nephrotoxicity.6,7
Amikacin efficacy may be improved by inhaled administration, and its encapsulation within liposomes can improve delivery to the lung and most importantly into intracellular spaces where MAC bacteria evade host defences and antibiotics.8 In in vitro studies, Amikacin Liposomal Inhalation Suspension (ALIS*); has demonstrated penetration into macrophages and biofilms, where MAC bacteria hide,8,9 and penetration and dispersion within the lung.10
In the Phase III CONVERT study of ALIS in 224 patients with a long history of MAC-PD (in most enrolled patients) who had not achieved culture conversion with oral guideline-based therapy alone (GBT; n=112), 29.0% of patients achieved culture conversion with the addition of ALIS to GBT compared with 8.9% on GBT alone (p<0.0001).11,12 Of these patients (n=65), culture conversion was maintained during therapy and following withdrawal of all therapy at 12 months post-conversion (63.1% n=41/65). Following 12 months of treatment with ALIS plus GBT or GBT alone, all treatment was stopped. At 3 months with no anti-MAC treatment, 16.1% of patients who had been treated with GBT plus ALIS remained culture negative compared with no patients who had received GBT alone (p=0.0001).12
Dr Lorent presented case studies in MAC-PD. These explored the decision points for treatment initiation including:
- Effective medical history to reveal risk factors for disease progression, e.g. low body mass index (BMI)13
- Treatment approaches in patients with or without risk factors for disease progression, (e.g. cavitary lesions, positivity for acid-fast bacilli1)
- Prompt and appropriate timing of treatment interventions to prevent disease progression rather than watch and wait.14
However, starting therapy for NTM-PD or MAC-PD is often hindered by the emergence of common AEs and can lead to treatment withdrawal or modification and treatment failure.15,16 Professor Aliberti considered the burden of the disease itself (e.g. weight loss, disease progression) against the burden of treatment itself (e.g. administration of ≥3 drugs for up to 18 months plus regular monitoring). Patients indicated that drug side effects are a major challenge for them17 and Professor Aliberti presented an overview of his clinical practice with respect to monitoring for AEs and his management strategies including enhanced evaluation or treatment discontinuation.
Inhaled medications present different AE challenges compared with systemically administered drugs, and Professor Aliberti presented the most common AEs with ALIS in the Phase III CONVERT study, which were, unsurprisingly, respiratory in nature: dysphonia (45.7% vs 0.9% for GBT alone), cough (37.2% vs 15.2%), dyspnoea (21.5% vs 8.9%) and oropharyngeal pain (10.8% vs 1.8%).11 He presented data from Swenson et al. that provide practical strategies to overcome AEs with ALIS.18 Strategies include mitigating dysphonia with antitussives, lozenges and warm water, and managing increased sputum development with improved airway clearance techniques. Of course, management of AEs needs to go beyond simple prescription of medications or use of other non-pharmacological aids, and Professor Aliberti presented the key role for the multidisciplinary team in supporting patients through their MAC-PD/NTM-PD treatment journey and the valuable support that can be gained from patient support groups.
CME-accredited satellite symposium ‘Patient identification, selection, and timing of antimicrobial treatment in the context of year 2020 ERS/ATS/ESCMID/IDSA guideline-based therapy for MAC lung disease: new guidance for treatment of high risk and refractory disease’ supported by an educational grant from Insmed.
This CME-accredited symposium explored the guidelines developed in 20201 alongside high-risk patient groups for NTM-PD; notably, those with bronchiectasis and chronic obstructive pulmonary disease (COPD). Professor Lange, who co-chaired the session with Dr Charles Daley, introduced the guidelines and the NTM species of interest, which was later expanded by Dr Daley who gave an overview of the guideline development and recommendations. Professor Aliberti presented the risk factors for NTM-PD and the pivotal roles that non-cystic fibrosis bronchiectasis and COPD have in the risk of developing NTM-PD. Professor Rachel Thomson went on to provide insight into ALIS with a focus on the mechanism of NTM infection within the macrophage, the role of the liposome in facilitating cellular entry, and the functional aspects of ALIS delivery and how this translated into Phase II and III studies.19 The symposium concluded with a series of case studies for panel discussion.
The role of patient and disease morphology and immunological profile in NTM-PD
Apart from the symposia, there was a focus on NTM-PD from the morphological and immunological perspective, and how these relate to patient phenotype and prognosis, as well as an exploration of patient treatment and management of AEs.
Dr Emilie Catherinot presented a study that explored the morphological and immunological characteristics of patients with NTM-PD versus matched controls.20 It is known that many patients with NTM-PD present with a characteristic morphotype of taller than average height, with a low BMI and thoracic skeletal abnormalities.21
In this study of patients with NTM-PD (n=50), most were women (90%) with a mean age of 65 years. Overall, the NTM-PD cohort was taller and thinner compared with the control group with an increased frequency of thoracic wall abnormalities and mitral valve prolapse versus matched controls (e.g. mean BMI of 20.37 vs 25.63 kg/m2) and 44 patients had nodular bronchiectasis.
Evaluation of their immunological parameters indicated that patients with NTM-PD had diminished memory B-cell levels and reduced levels of interleukin-12 (IL-12) and tumour necrosis factor alpha (TNF-α) compared with controls, but seemingly no underlying genetic abnormalities. Stimulation of whole blood or peripheral blood mononuclear cells (PBMCs) from these patients with NTM mitogens triggered a lower production of interferon gamma, IL-12 and TNF compared with controls.
Dr Catherinot suggested a role for adipokines in this reduction, in that reduced leptin expression increases adiponectin levels to bias a T helper 2 cell immune phenotype that enhances phagocytosis and induction of interleukin-1 receptor antagonist, IL-10 and suppression of TNF expression. She further suggested that mitral valve prolapse, scoliosis and pectus excavatum, as connective tissue disorders, are likely to be associated with transforming growth factor beta pathways, which in mouse models are known to increase host susceptibility to MAC and M. tuberculosis. Her summary suggested that these data indicate a broader immunodeficiency mechanism underlying NTM-PD, perhaps as a result of an undetected genetic disorder that affects the cooperation of T and B cells or the presentation of major histocompatibility complex to detect and respond to NTM infection. Given the homogeneity of patients in this study and the often-encountered adult idiopathic bronchiectasis patient, whole exome sequencing is now underway.
Understanding in which patients diagnosed with NTM-PD the disease will progress is challenging. It has been shown in a study that when left untreated, nodular bronchiectatic MAC-PD will progress over time,22 requiring that diagnosed patients are carefully monitored in order to start treatment promptly at the point of change in computed tomography scans. The 2020 NTM-PD guidelines1 recommend prompt treatment for patients who meet the diagnostic criteria for disease and have positive acid-fast bacilli sputum smears and/or cavitary lung disease. However, the decision to treat NTM-PD is a personal one between clinician and patient and any data that can support both to make an informed choice is positive.
Dr Marty et al. explored NTM-PD disease progression from the perspective of the immune system23 and whether aspects of cellular immunity could act as surrogate markers to understand which patients are at risk of disease progression. Using flow cytometry to detect activation of induced markers on T cells in PBMCs from NTM-PD patients after challenge with mycobacteria-specific peptide pools (MTB300), purified protein derivative or control, the authors were able to explore the expression of T cells in response to NTM infection. In this study, patients with NTM-PD (n=14, of whom 6 had progressive disease) were compared with 12 tuberculosis (TB)-unexposed patients. The proportion of CD3+, CD4+, and CD8+ T cells co-expressing activated induced markers (CD25+, CD134+) was evaluated. In PBMCs from patients with NTM-PD, significant differences in CD25+ CD134+ co-expression were observed compared with that from TB-unexposed patients (p<0.005). Similarly, CD8+ CD25+ CD134+ T cells were significantly different in patients with progressive NTM-PD compared with those with non-progressive disease (p<0.01). The authors suggested that immuno-profiling of patients might distinguish between patients with NTM-PD and unexposed controls, as well as highlight which patients will have progressive disease over those with non-progressive disease.
It has been recognised that NTM and TB share similar clinical presentations for pulmonary and extrapulmonary disease so that there is the potential to overdiagnose TB at the expense of overlooking NTM infection or NTM-PD/TB co-infection.24 In a retrospective study of surgical lung resections excised from 38 patients with confirmed TB, it was noted that all patients also had presence of slow-growing NTM species.25
Morphological evaluation of the lung post-infection suggested that differential diagnosis between NTM and TB was possible. Histologically, patients with mycobacteriosis had abundant lymphoid infiltration of the parenchyma and formation of macrophage granulomas typical of M. avium (22.5%) and M. intracellulare. Patients with M. kansasii and M. xenopi had characteristic lymphoid-histiocytic infiltration of the walls of the parenchyma (30% and 27.5%, respectively). The authors suggested that clinically important changes within the lung can be identified within surgical material for patients with pulmonary TB and should be employed for differential diagnosis of TB and NTM-PD.
Practical considerations for determining the point of treatment for NTM-PD and the impact of AEs
Patients presenting with culture-positive NTM samples do not always qualify for antibiotic treatment under the current guideline recommendations.1 Understanding who needs treatment for active infection and whose identified sputum cultures are not currently clinically relevant (requiring active monitoring) remains difficult. In a retrospective chart review study (2018–2019) conducted in the UK at a respiratory clinic, 93 patients identified with sputum or bronchial lavage positive for NTM were evaluated.26
In these patients, the most common species of NTM identified was MAC in line with other published data,26,27 followed by M. gordonae. Of the patients identified as NTM culture positive, 25% with culture-positive sputum or bronchial lavage received treatment in line with British Thoracic Society guidelines;28 the remainder were considered to have infection that was not clinically relevant and were not treated. Of patients with a positive NTM culture, 61% had underlying respiratory conditions such as bronchiectasis (n=35), COPD (n=22) or asthma (n=17). The author suggested that determining NTM-PD over patients with NTM organisms that are not clinically relevant or those who do not yet meet the threshold for clinically relevant disease and require active monitoring is challenging and, in this study, the diagnostic accuracy of the 25% of patients treated for disease needs to be determined. Dr Maung Maung Mint suggested that there is also a need to establish clear patient pathways for ongoing investigation for patients. In particular, pathways should distinguish between those patients who are NTM culture positive for NTM but may be considered as colonised versus those who have NTM infections to ensure that no opportunities to treat NTM-PD are missed.
AEs are an important consideration when treating patients with NTM-PD. AEs with combination therapy for NTM-PD are common and can facilitate non-adherent to therapy or stop altogether. In the study by Vladimirova et al., AEs following treatment for NTM-PD were evaluated.29 In this study, 112 patients with NTM-PD received three oral antibiotics and therapy correction (e.g. decreased dosing frequency) was required in 30.6% of patients due to AEs, with 13.5% stopping treatment due to severe AEs. AEs were observed in 33% of patients overall, with administration of rifabutin responsible for most AEs (Table 1).
The most common AEs were:
- Gastrointestinal disorders: 34%, particularly in patients receiving protionamide, and 28% each for rifampicin and macrolides
- Hepatitis: 17% with each of rifabutin, rifampicin and fluoroquinolone
- Cardiotoxic effects: 12% with each of protionamide, macrolides and fluoroquinolones
- Ototoxicity: 11% with aminoglycosides
- Optic neuritis: 5% with ethambutol.
Table 1. Proportion of patients with AEs as a result of antibiotics.29
|
Antibiotic |
Patients with an AE, % |
|
Rifabutin |
46 |
|
Protionamide |
42 |
|
Isoniazid |
31 |
|
Macrolides |
22 |
|
Fluoroquinolones |
23 |
|
Aminoglycosides |
22 |
|
Rifampicin |
20 |
|
Pyrazinamide |
23 |
This study reiterated the complexity of NTM-PD therapy and suggested that the choice of antibiotic should also consider potential AEs in individual patients. When AEs emerge, it is important to explore whether treatment should be modified or terminated.
In summary
It is clear that the profile of NTM and NTM-PD within international congresses such as the ERS is increasing, as NTM-PD as a rare disease emerges from the shadows. In 2021, the focus for NTM-PD at the ERS Annual Congress has been about underlying mechanisms of disease, with emphasis on involvement of the immune system.
*ALIS known in Europe as ARIKAYCE® liposomal 590 mg nebuliser dispersion30
References:
- Daley CL, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020;56:2000535.
- Angrill J, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med 2001;164:1628–32.
- Fukushima K, et al. Long-term treatment outcome of progressive Mycobacterium avium complex pulmonary disease. J Clin Med 2020;9:1315.
- Diel R, et al. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis 2018;18:206.
- Jo K-W, et al. Treatment outcomes of refractory MAC pulmonary disease treated with drugs with unclear efficacy. J Infect Chemother 2014;20:602–6.
- Kobashi Y, et al. A double-blind randomized study of aminoglycoside infusion with combined therapy for pulmonary Mycobacterium avium complex disease. Respir Med 2007;101:130–8.
- Peloquin CA, et al. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis 2004;38:1538–44.
- Chalmers JD, van Ingen J, van der Laan R, Hermann J-L. Liposomal drug delivery to manage nontuberculous mycobacterial pulmonary disease and other chronic lung infections. Eur Respir Rev 2021; 30:210010.
- Zhang J, et al. Amikacin Liposome Inhalation Suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol 2018;9:915.
- Olivier KN, et al. Airway deposition and retention of liposomal amikacin for inhalation in patients with pulmonary nontuberculous mycobacterial disease. Presented at the American Thoracic Society 2016 International Conference; San Francisco, CA, USA. Poster A3732.
- Griffith DE, et al. Amikacin Liposome Inhalation Suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med 2018;198:1559–69.
- Griffith DE, et al. Amikacin Liposome Inhalation Suspension for refractory Mycobacterium avium complex lung disease: sustainability and durability of culture conversion and safety of long-term exposure. Chest 2021;160:831–42.
- Kwon YS, et al. Treatment of Mycobacterium avium complex pulmonary disease. Tuberc Resp Dis (Seoul) 2019;82:15–26.
- Jhun BW, et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J 2020;55:1900798.
- Aliberti S, et al. Real-life evaluation of clinical outcomes in patients undergoing treatment for non-tuberculous mycobacteria lung disease: a ten-year cohort study. Resp Med 2020;164:105899.
- Abate G, et al. Variability in the management of adults with pulmonary nontuberculous mycobacterial disease. Clin Infect Dis 2021;72:1127–37.
- Shteinberg M, et al. What is important for people with nontuberculous mycobacterial disease? An EMBARC-ELF patient survey. ERJ Open Res 2021;7:00807-2020.
- Swenson C, et al. Clinical management of respiratory adverse events associated with Amikacin Liposome Inhalation Suspension: results from a patient survey. Open Forum Infect Dis 2020;7:ofaa079.
- Chalmers JD, et al. Liposomal drug delivery to manage nontuberculous mycobacterial pulmonary disease and other chronic lung infections. Eur Respir Rev 2021;30:210010.
- Catherinot E, et al. Pulmonary non-tuberculous mycobacterial infection in patients without predisposing condition, morphological and immunological overview of a French cohort. Presented at the European Respiratory Society 2021 Annual Congress (virtual). ePoster 4207, Abstract 536986.
- Prevots DR, et al. Epidemiology of human pulmonary infection with non-tuberculous mycobacteria: a review. Clin Chest Med 2015;36:13–34.
- Park TY, et al. Natural course of the nodular bronchiectatic form of Mycobacterium avium complex lung disease: long-term radiologic change without treatment. PLoS One 2017;12:e0185774.
- Marty P, et al. Immune profiling to differentiate progressive from non-progressive non-tuberculosis mycobacteria lung disease: a pilot study. Presented at the European Respiratory Society 2021 Annual Congress (virtual). ePoster 3088, Abstract 535865.
- Lin C-K, et al. Incidence of nontuberculous mycobacterial disease and coinfection with tuberculosis in a tuberculosis-endemic region. Medicine (Baltimore) 2020;99:e23775.
- Lepeha L, et al. Morphological features of non-tuberculosis mycobacteriosis in patients operated on for lung tuberculosis. Presented at the European Respiratory Society 2021 Annual Congress (virtual). ePoster 3084, Abstract 535857.
- Maung Maung Myint Y, et al. The non-tuberculosis pulmonary disease experience in a general respiratory clinic in the UK. Presented at the European Respiratory Society 2021 Annual Congress (virtual). ePoster 3092, Abstract 535870.
- Vande Weygaerde Y, et al. Clinical relevance of pulmonary non-tuberculous mycobacterial isolates in three reference centres in Belgium: a multicentre retrospective analysis. BMC Infect Dis 2019;19:1061.
- Haworth CS, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017;72(Suppl. 2):iii1–ii64.
- Vladimirova E, et al. Adverse reactions in the treatment of patients with non-tuberculosis pulmonary mycobacteriosis. Presented at the European Respiratory Society 2021 Annual Congress (virtual). ePoster 3085, Abstract 535861.
- ARIKAYCE liposomal 590 mg nebuliser dispersion. Available at: https://www.ema.europa.eu/en/documents/product-information/arikayce-liposomal-product-information_en.pdf Accessed October 2021.
NTM: Convert, Cure or Fail – the treatment journey for NTM-PD
NTM: Initiating treatment for NTM-PD - putting the patient at the heart of the matter
NTM: Screening for NTM-PD: How to get ahead of the curve
NTM: Risks for NTM-PD that run under the radar