Non-tuberculous mycobacteria (NTM)
Non-tuberculous mycobacteria, or NTM, cause rare lung infections, mainly in people who have underlying lung conditions or who have a problem with their immune system. These infections are sometimes known as NTM pulmonary disease (NTM-PD).
NTM are ubiquitous in the environment in both soil and water sources.1–3 In vulnerable patients, such as those with pre-existing underlying lung conditions, low body mass index (<18.5 kg/m2), thoracic skeletal abnormalities, gastroesophageal reflux disorder (GERD) or immunodeficiencies, NTM can cause a potentially debilitating disease called NTM-PD, which can be difficult to diagnose and treat.4,5 In some areas, NTM-PD is on the increase, with the incidence of pulmonary NTM isolates in the UK rising from 4.0/100,000 in 2007 to 6.1/100,000 in 2012.6 In Germany, the incidence of NTM-PD in 2016 was even higher at 15.3 cases per 100,000.7
Among NTM, the Mycobacterium avium complex (MAC) is the most common disease-causing mycobacteria. Currently, MAC consists of twelve species8 and the two most clinically relevant species are M. avium and M. intracellulare.9 MAC is the most commonly isolated NTM – 47%.10 Other common species which cause NTM-PD include M. abscessus, M. kansasii and M. xenopi.11
Disease Background
Non-tuberculous mycobacteria (NTM) can cause serious disease in at-risk patients and in vulnerable people can cause a difficult-to-treat pulmonary infection – NTM-PD.
NTM-PD is a potentially debilitating pulmonary disease.12–16 The symptoms of NTM-PD are non-specific and include chronic cough, fatigue, weight loss and low-grade fever. They are typically similar to those of underlying lung conditions such as bronchiectasis and chronic obstructive pulmonary disease (COPD), which complicates diagnosis.5
NTM-PD is treated with guideline-based therapy (GBT)11 which consists of multidrug regimens that need to be administered for up to 12 months beyond culture conversion in many cases.
Patients most at risk of developing NTM-PD include individuals:4
- with underlying lung conditions such as bronchiectasis or COPD (relative risk [RR] 44.0–187.5 and 2.0–10.0, respectively)
- with low BMI (<18.5 kg/m2) (RR 9.1)
- with thoracic skeletal abnormalities (RR 5.4)
- on immunomodulatory/immunosuppressant therapies or using steroids (RR 1.3–2.2 and 1.6–8.0, respectively)
- with gastroesophageal reflux disease (GERD) (RR 1.5–5.3)
Burden of Disease
NTM-PD is a chronic, potentially debilitating disease with a substantial burden on patients.
Patients with NTM-PD typically experience accelerated decline in lung function, increased morbidity and mortality and a substantial reduction in their health-related quality of life.12–15,17 All-cause mortality in patients with NTM-PD is up to four times higher than the general population, independent of other factors,16,18 with an average estimated 5-year mortality of 27% for MAC infections and 19.5% for M. abscessus infections.19,20 M. xenopi has a higher 5-year mortality; studies have indicated a 5-year mortality of 43% and 51%,11 but the mortality rate for the very aggressive M. kansasii appears to the highest; M. kansasii has a high mortality rate with an estimated 1-year mortality of 43% according to one study.21
NTM-PD also increases the risk of:
- subsequent lung infections (e.g. aspergillosis)22
- exacerbations4
and has been implicated in the development of lung cancer23,24 and atrial fibrillation25
Mode of Disease
NTM species invade macrophages and accumulate in biofilms to evade host defences, making them challenging to treat.
When NTM species are inhaled, they can cause infections which can be difficult to treat with antibiotics. Left untreated NTM-PD may progress21,26
- Once inhaled, NTM species invade macrophages and accumulate in biofilms27–31
- This allows NTM to evade host defences and many antibiotics27,29,30
- NTM alters normal macrophage function to resist bactericidal responses and changes the inflammatory response of the host, facilitating unhindered bacterial reproduction28,32
- NTM then triggers apoptosis, releasing bacteria to infect adjacent macrophages32–34
Why Treat?
Certain patients may be at higher risk of progressive NTM-PD, so don’t wait to treat these patients – take action.
The decision to treat NTM-PD is influenced by the:5
- severity of the disease
- risk of disease progression
- presence of comorbidities
- goals of treatment
Patients with specific characteristics are likely to have potentially progressive NTM-PD, so don’t wait to treat patients with risk factors. When untreated, nodular bronchiectatic MAC-PD has been shown in one study to progress over 6 years in 97.5% of patients,26 whilst in another study 43% of those with M. kansasii are likely to progress within 1 year of diagnosis with a low median survival if treatment was not started promptly.21
Characteristics that put patients diagnosed with NTM-PD at high risk of disease progression are:9,11
- more virulent NTM species - treat those of high clinical relevance such as MAC ( avium and M. intracellulare have been shown to be clinically relevant in 63% and 88% of patients, respectively) and M. kansasii (78%)
- positive acid-fast bacilli test
- presence of lung cavities
- low BMI
The primary goals of therapy for NTM-PD are:5
- sustained culture conversion of sputum on treatment
- improvement of symptoms
- potential radiological improvements
International NTM management guidelines recommend early treatment, stating that the benefits outweigh the risks, especially in the context of the presence of acid-fast bacilli and/or cavitary lung disease.11 Before treating NTM-PD, establish the goals of therapy for your patient and follow the guidelines for treatment.
Unmet Need
Currently, treatment options and outcomes for NTM-PD are poor. Many antibiotics cannot penetrate macrophages and biofilms, and patients on macrolide monotherapy are at risk of developing macrolide resistance.
Once identified, treating NTM-PD is essential particularly where virulent mycobacterial species are identified, but treatment options and outcomes are often poor, and patients who fail treatment for NTM-PD have limited options. Depending on the NTM species, treatment success rates for NTM-PD vary from 32% to 80% (32% M. xenopi, 41%, MABC, 66% for MAC, 80% M. kansasii),35,36 and for patients who have failed on oral GBT, their chances of a favourable outcome with subsequent treatments can be as low as 16% regardless of the treatment regimen.37
NTM species are able to invade macrophages and accumulate in biofilms,27–31 which many antibiotics are unable to penetrate. This enables bacteria to evade these antibiotics, making NTM-PD challenging to treat.29,30,35,
Many at-risk patients will receive long-term macrolide monotherapy to prevent exacerbations of underlying disease,38 increasing the risk of macrolide-resistant NTM-PD. Whilst macrolide monotherapy may help reduce the risk for NTM-PD in patients with cystic fibrosis,39 macrolide monotherapy is one of the major predispositions for macrolide-resistant MAC infection.40 Macrolide resistance is associated with increased mortality and is a threat to many at-risk patients. While the average estimated all-cause 5-year mortality for patients with MAC-PD is 27%,19 in patients with macrolide resistance, mortality rates are even worse at over 45% over 5 years.41
Featured content

Article
Read time: 5 mins
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) with antimicrobial agents offers the possibility of cure. In patients who meet the clinical, radiographical and microbiological diagnostic criteria for NTM-PD.

Article
NTM: Impact of non-tuberculous mycobacteria (NTM) on at-risk patients
Read time: 5 mins
Non-tuberculous mycobacteria (NTM) can cause serious pulmonary disease in at-risk patients, which can have a significant impact on health-related quality of life, morbidity and mortality, and increase disease progression in patients

Article
NTM: Understanding the risk factors that underlie NTM-PD
Read time: 12 mins
Understanding the risk factors that are common in patients with NTM-PD provides a valuable insight into patients who might benefit from testing for NTM to rule out disease and, if NTM infection is present, what might be the appropriate course of action.
Find out more about NTM-PD
Explore which patients to treat, and when a decision to treat has been made, how to do this in line with current guideline recommendations
Additional useful information on NTM
A variety of external resources which provide additional useful information on non-tuberculous mycobacterial pulmonary disease. The content in the links provided herein are from third party websites and are not owned or created by Insmed Incorporated. Please refer to the third party websites for their respective terms of service.
EMBARC
NTM Net
European Lung Foundation
ERS CME NTM module
References:
- Nishiuchi Y et al. Front Med 2017;4:27.
- Falkinham JO Clin Chest Med 2015;36:35–41.
- Falkinham JO. J Appl Microbiol 2009;107:356–67.
- Prevots DR et al. Clin Chest Med 2015;36:13–34.
- Griffith DE et al. Am J Respir Crit Care Med 2007;175:367–416.
- Shah NM et al. BMC Infect Dis 2016;16:195.
- Ringshausen FC et al. Int J Infect Dis. 2021;104:398–406.
- Van Ingen J et al. Int J Syst Evol Microbiol 2018;68:3666–77.
- Zweijpfenning SMH et al. Semin Respir Crit Care Med 2018;39:336–42.
- Hoefsloot W et al. Eur Respir J 2013;42:1604–13.
- Daley CL et al. Eur Respir J 2020;56:2000535.
- Park HY et al. Chest 2016;150:1222–32.
- Kobayashi T et al. J Clin Tuberc Other Mycobact Dis 2018;11:17–21.
- Lee MR et al. PLoS One 2013;8:e58214.
- Huang CT et al. Int J Tuberc Lung Dis 2012;16:539–45.
- Marras TK et al. Respir Med 2018;145:80–8.
- Fleschner M et al. Int J Tuberc Lung Dis 2016;20:582–7.
- Diel R et al. Eur Resp J 2017;49:1602109.
- Diel R et al. BMC Infect Dis 2018;18:206.
- Marras TK et al. Emerg Infect Dis 2017;23:468–76.
- Liu C-J et al. Respir Med 2019;151:19–26.
- Yeung MW et al. Respirology 2016;21:1015–25.
- Tamura A et al. Open Respir Med J 2016;10:20–8.
- Taira N et al. Am J Case Rep 2018;19:748–51.
- Park CS et al. Sci Rep 2019;9:15503.
- Park TY et al. PLoS One 2017;12:e0185774.
- Awuh JA et al. Cell Mol Life Sci 2017;74:1625–48.
- Ganbat D et al. BMC Pulm Med 2016;16:19.
- Chakraborty P et al. Microbiol Cell 2019;6:105–22.
- McGarvey et al. Clin Chest Med 2002;23:569–83.
- Esteban J et al. Front Microbiol 2018;8:Article 2651.
- Chiplunkar SS et al. Future Microbiol 2019;14:293–313.
- Sousa S et al. Microorganisms 2019;7:113.
- Lee K-I et al. Sci Rep 2016;6:37804.
- Diel R et al. Chest 2017;152:120–42.
- Diel R et al. Chest 2018;153:888–91.
- Jo K-W et al. J Infect Chemother 2014;20:602–6.
- Smith D et al. BMJ Open 2020;7:e000489.
- Coolen N et al. J Cyst Fibros 2015;14:594–9.
- Griffith DE, et al. Curr Opin Infect Dis 2012;25:218–27.
- Moon SM et al. Antimicrob Agents Chemother 2016;60:6758–65.
Benefits of early treatment initiation in non-tuberculous mycobacterial pulmonary disease (NTM-PD)
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) with antimicrobial agents offers the possibility of cure.1 In patients who meet the clinical, radiographical and microbiological diagnostic criteria for NTM-PD, the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for NTM-PD recommend initiation of treatment rather than watchful waiting.1 Initiation is especially important in the context of positive acid-fast bacilli sputum smears and/or cavitary lung disease1as there may be an increased rate of progression and poor treatment outcomes if treatment is delayed.1
Evidence of disease progression in untreated MAC-PD
Several studies have shown that most patients diagnosed with Mycobacterium avium complex pulmonary disease (MAC-PD) have progressive disease resulting in the need for antibiotic treatment.2,3 In a recent study of 488 newly diagnosed patients at the Asan Medical Center in South Korea, 305 (62.5%) patients showed progressive MAC-PD resulting in treatment initiation within 3 years of diagnosis.2 Similarly, in another study of 40 untreated patients with the nodular bronchiectatic form of MAC-PD (most with minimal symptoms), who underwent serial chest computed tomography (CT) scans for a minimum of 4 years, 39 (97.5%) experienced disease progression with a significant increase in overall CT score.3 It is noted in the 2020 NTM-PD guidelines that some subgroups (minimal nodular/bronchiectatic disease) may be safely, but regularly, followed without antimicrobial therapy; however, those with cavity disease should always receive prompt antibiotic treatment.1
Factors influencing the decision to initiate treatment
The decision to treat may be influenced by both host factors and infecting bacterial species. Certain factors like cavitary disease and low body mass index have been associated with progressive disease and may necessitate earlier consideration of antibiotic treatment.2 In very frail patients with very mild nodular bronchiectatic disease, the balance between efficacy and tolerability may favour watchful waiting.1
The clinical relevance of NTM varies significantly between species (Figure 1) and may also differ geographically.1,4 For example, species such as M. gordonae have low pathogenicity and rarely cause disease in humans, whereas M. kansasii is highly pathogenic.1,4
Figure 1. Clinical relevance (the percentage of patients with isolates of these species that meet the ATS/IDSA diagnostic criteria) of non-tuberculous mycobacterial species. M., Mycobacterium. Adapted from Zweijpfenning (2018).5
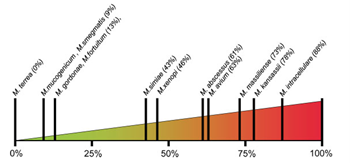
The most common NTM pathogens include MAC, M. kansasii and M. xenopi among the slowly growing NTM and M. abscessus among the rapidly growing NTM.1
Meeting the guideline-recommended diagnostic criteria for NTM-PD
Diagnostic criteria within the guideline is based on:
- Clinical symptoms e.g. worsening of symptoms of underlying lung conditions, or onset of new, persistent symptoms in patients at risk of NTM-PD e.g. haemoptysis, weight loss, fatigue
- Radiological findings on X-ray or hight-resolution CT scan such as nodular or cavitary opacities
- Microbiological findings from a) at least two expectorated sputum or b) positive culture from at least one bronchial wash or lavage or c) positive culture for NTM and biopsy from transbronchial or lung biopsy plus one or more culture positive sputa or bronchial washing.
Patients suspected of having NTM-PD who do not meet the diagnostic criteria should be actively managed and followed with serial CT scans until the diagnosis is firmly established or excluded and should start or continue recommended techniques such as airway clearance.6
The decision to initiate antibiotic treatment
NTM-PD is associated with diminished health-related quality of life that correlates with severity of lung impairment;7 antimicrobial treatment may be associated with improvement.8
NTM-PD treatment decisions are often difficult and require experience in managing the disease. This can mean that it may be necessary for a peer consultation or referral to a pulmonologist or infectious disease specialist with experience in NTM-PD.1,9 The virulence and potential for progressive disease must be evaluated once the NTM species is identified in order to determine treatment. In the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for NTM-PD for example, it is recommended that for species of low pathogenicity such as M. gordonae, treatment is only indicated if repeated positive cultures over several months are observed, along with strong clinical and radiological evidence of disease whereas in many patients only one positive M. kansasii sputum culture may be required in order to initiate treatment.1 Similarly, clinically significant MAC-PD is unlikely in patients who have a single positive sputum culture during the initial evaluation but can be as high as 98% in those with ≥2 positive cultures.1 Two or more MAC-positive cultures indicate active MAC infection requiring a treatment decision, whereas for patients identified with M. kansasii, treatment should be initiated as soon as a single positive culture is obtained.1
Regardless of the infecting organism, the decision to initiate antibiotic treatment should be individualised considering the patient’s symptoms, the pathogenicity of the organism, radiological findings, microbiological results and importantly, the patient’s wish and ability to receive treatment as well as the goals of therapy.1 Any treatment decision should include a discussion with the patient that outlines the potential side-effects of antimicrobial therapy, the uncertainties surrounding the benefits of antimicrobial therapy and the potential for recurrence including reinfection (particularly in the setting of nodular/bronchiectatic disease).1 Guidelines recommend regular sputum cultures and routine monitoring to assess disease progression.1
 Following treatment initiation, sputum specimens should be obtained for culture every 1 to 2 months to document when sputum cultures become negative and to survey for the appearance of other organisms. 1
Following treatment initiation, sputum specimens should be obtained for culture every 1 to 2 months to document when sputum cultures become negative and to survey for the appearance of other organisms. 1
Clinical and radiographical assessments should be performed alongside the microbiological assessments to determine if the patient is responding to therapy.1
Retrospective studies have shown that most patients with MAC-PD who convert on treatment do so within 6 months of starting treatment.11–13
If you decide not to initiate antibiotic treatment, an active monitoring plan is recommended by the guidelines.1 Study data suggest that untreated NTM-PD could progress.2,3
References:
- Daley Cl, et al. Clin Infect Dis 2020;71:e1–e36.
- Hwang JA, et al. Eur Respir J 2017;49:1600537.
- Park TY, et al. PLoS One 2017;12:e0185774.
- van Ingen J, et al. Thorax 2009;64:502–6.
- Zweijpfenning SMH, et al. Semin Respir Crit Care Med 2018;39:336–42.
- Lipman M, et al. BMJ Open Respir Res 2020 ;7 :e000591
- Mehta M, Marras TK. Respir Med 2011;105:1718–25.
- Czaja CA, et al. Ann Am Thorac Soc 2016;13:40–8.
- Ryu YJ, et al. Tuberc Respir Dis 2016;79:74–84.
- Lee MR, et al. Clin Microbiol Infect 2015;21:250.e1–250.e7.
- Furuuchi K, et al. Chest 2020;157:1442–5.
- Koh WJ, et al. Eur Respir J 2017;50:1602503.
- Moon SM, et al. Eur Respir J 2019;53;1801636.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
,
,
Impact of non-tuberculous mycobacteria (NTM) on at-risk patients
Non-tuberculous mycobacteria (NTM) can cause serious pulmonary disease in at-risk patients, which can have a significant impact on health-related quality of life, morbidity and mortality, and increase disease progression in patients with structural lung diseases. Understanding who is at risk can facilitate earlier diagnosis and treatment, which is crucial in preventing disease progression and lung function decline.
Non-tuberculous mycobacteria (NTM) are opportunistic infections that can cause infection at a wide range of body sites in patients who have underlying disease or are immunosuppressed.1 Inhalation of some NTM species in vulnerable people can cause non-tuberculous mycobacterial pulmonary disease (NTM-PD)2. NTM-PD can be caused by a variety of mycobacterial species, the most common of which is the Mycobacterium avium complex (MAC), which comprises two main species M. avium and M. intracellulare. In one study of 62 centres in 30 countries of 18,418 isolates MAC-PD accounted for 47% of incidences of NTM-PD.3
How does NTM-PD have an impact on quality of life?
NTM-PD can be a significant burden on patients. Patients with NTM-PD, including MAC-PD, may have reduced lung function, increased morbidity and mortality, and reduced health-related quality of life (HRQoL) compared with the general population.3,4–11
All-cause mortality in patients with NTM-PD can be up to four times higher than the general population, independent of other factors.10–12 For MAC-PD, studies showed a pooled estimate of five-year all-cause 5-year mortality of 27%.2 NTM-PD can also cause a significant reduction in patients’ lung function.7–9 Patients with NTM-PD have been shown to experience a more substantial reduction in forced expiratory volume in 1 sec (FEV1) compared with those without NTM-PD.8 In one study where patients with mild disease were considered not to require treatment, chronic NTM infection caused a substantial decline in lung function over time.7 NTM-PD is associated with a lower HRQoL compared with the general population, with these patients demonstrating higher scores using the St. George’s Respiratory Questionnaire (SGRQ) and lower scores using the Medical Outcomes Study 36-item Short Form Survey (SF-36).4,13 In one study, SGRQ scores in patients with NTM-PD were over 25 points worse compared with normal values.13 Another study showed that patients eventually requiring treatment for their NTM-PD had worsening SGRQ scores, suggesting an association between disease progression and lower HRQoL.4
Who is most at risk of NTM-PD?
Understanding who is at increased risk of NTM-PD can help in early recognition and diagnosis of disease. High-risk groups include tall, elderly women with a low body mass index (BMI) and abnormalities of the skeleton for example, conditions such as abnormal spinal curvatures (scoliosis, kyphosis) and structural abnormality of the chest where the sternum is pressed inward (pectus excavatum) – so-called Lady Windermere syndrome – patients with underlying lung conditions such as bronchiectasis and chronic obstructive pulmonary disease (COPD), and immunocompromised and immunosuppressed patients; exposure to NTM species is much more likely to cause disease in these groups.6,14–16
Patients with low BMI
There is an association between NTM-PD and marfanoid characteristics of elderly female patients who are taller than average with low body weight, as well as those with thoracic skeletal abnormalities; low body weight alone increases risk of NTM-PD by three-fold and thoracic abnormalities five-fold.17,18 In patients with NTM-PD, low BMI (<18.5 kg/m2) has been associated with the presence of multiple NTM isolates as well as a lower chance of treatment success.4,5 In addition, patients with lower BMI are more likely to fail treatment for their NTM-PD.4
Transplant recipients
NTM can also cause disease in immunosuppressed patients who are recipients of organ or stem cell transplants.6 Rates of NTM infections in lung transplant recipients are particularly high, and have been shown to increase post-transplant mortality, with an estimated 5-year mortality of 50%.6
Patients with structural lung diseases
NTM can cause pulmonary disease in patients with pre-existing underlying lung conditions, such as those with bronchiectasis (44.0–187.5 increased risk) and COPD (2.0–10.0 increased risk).15,16 Infection and inflammation caused by NTM in such patients can lead to deterioration of pulmonary function, causing faster disease progression compared with patients without NTM-PD.5 In one study, the presence of multiple NTM isolates in patients with COPD has been associated with a greater decline in FEV1 as well as an increase in exacerbations requiring hospitalisation compared with the absence of isolates.5
Act now for your at-risk patients
Early diagnosis and treatment of NTM-PD is crucial in preventing disease progression and declining lung function.19
NTM-PD is often misdiagnosed or diagnosed late, as symptoms of NTM-PD are similar to those of coexisting lung disease and may be present for more than 10 years before diagnosis; increasing testing for your at-risk patients may lead to a higher chance of spotting NTM-PD early.20,21 In a survey of 280 hospital-based physicians, the majority perceived NTM-PD as a significant factor for worsening respiratory function, increasing morbidity and hospitalisation in patients with bronchiectasis, although fewer perceived NTM-PD as having a significant impact on mortality.22 However, the mortality rate for NTM-PD and in particular MAC-PD is high (up to 27%),2 so recognising the risk of NTM-PD and testing your at-risk patients is important, as missing a diagnosis can lead to worse long-term outcomes for your patients, including increased risk of death.
At-risk patients with underlying lung conditions such as COPD or bronchiectasis may receive long-term macrolide monotherapy to prevent exacerbations of underlying disease.23 One study indicated that 42% of patients with bronchiectasis received macrolide monotherapy, however, macrolide monotherapy is not recommended in patients with NTM-PD because of the increased chance of macrolide resistance.22
Guidelines for managing bronchiectasis outline that any patient being considered for macrolide therapy for exacerbations should be screened for underlying NTM to rule out infection and protect antimicrobial susceptibility for NTM therapy.23
Currently, 68% of healthcare professionals do not test their patients for NTM-PD prior to initiating macrolide treatment, despite 87% perceiving these patients to be at particular risk of NTM infection.22 In fact, macrolide monotherapy is one of the major predispositions for macrolide-resistant MAC,24 so testing your at-risk patients for NTM-PD prior to initiating macrolide monotherapy is essential to reduce the emergence of macrolide-resistant NTM-PD and to increase the chance of cure. In patients with macrolide-resistant NTM-PD, treatment outcomes are poor, with an estimated all-cause 5-year mortality rate of 47%.25
References:
- Centers for Disease Control and Prevention. Nontuberculous mycobacteria (NTM) infections. https://www.cdc.gov/hai/organisms/nontuberculous-mycobacteria.html [Accessed March 2021]
- Diel R, et al. BMC Infect Dis 2018;18:206.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Kwak N, et al. BMC Pulm Med 2020;20:126.
- Huang CT, et al. Int J Tuberc Lung Dis 2012;16:539–45.
- Friedman DZP, et al. Transpl Infec Dis 2020;22:e13229.
- Park HY, et al. Chest 2016;150:1222–32.
- Kobayashi T, et al. J Clin Tuberc Other Mycobact Dis 2018;11:17–21.
- Lee MR, et al. PLoS One 2013;8(3):e58214.
- Marras TK, et al. Respir Med 2018;145:80–8.
- Fleshner M, et al. Int J Tuberc Lung Dis 2016;20:582–7.
- Diel R, et al. Eur Resp J 2017;49:1602109.
- Mehta M, et al. Resp Med 2011;105:1718–25.
- Aksamit TR, et al. Chest 2017;151:982–92.
- Andrejak C, et al. Thorax 2013;68:256–62.
- Prevots DR, et al. Clin Chest Med 2015;36:13–34.
- Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
- Axson EL, et al. Eur J Clin Microbiol Infect Dis 2019;38:117–24.
- Park TY, et al. PLoS One 2017;12:e0185774.
- Kotilainen H, et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Wagner D, et al. BMJ Open Respir Res 2020;7:e000498.
- Smith D, et al. BMJ Open Resp Res 2020;7:e000489.
- Griffith DE, et al. Curr Opin Infect Dis 2012;25:218–27.
- Moon SM, et al. Antimicrob Agents Chemother 2016;60:6758–65.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Understanding the risk factors that underlie NTM-PD
The ERS/ATS/ESCMID/IDSA 2020 NTM-PD guideline gives an overview of how to diagnose a patient for NTM-PD, relying on clinical, radiological and microbiological evaluations.1 But when a patient walks into the clinic, what is it about that individual that might prompt a clinician to consider testing for NTM?
Understanding the risk factors that are common in patients with NTM-PD provides a valuable insight into patients who might benefit from testing for NTM to rule out disease and, if NTM infection is present, what might be the appropriate course of action. Risk factors for NTM-PD include those inherent to the patient themselves (host risk factors), environmental risk factors (exposure), immunological risk factors, genetic risk factors and the presence of underlying lung conditions or disease.2
|
Factors increasing susceptibility of NTM-PDFactors |
Increased risk or prevalence of infection* |
|
Bronchiectasis |
44–187.53,4 |
|
Previous TB |
69.0–178.34,5 |
|
Low BMI |
9.13 |
|
Cystic fibrosis |
6.6–13.06,7 |
|
COPD (in receipt of ICS) |
29.14 |
|
COPD (no ICS) |
2.0–10.03 |
|
Thoracic skeletal abnormalities |
5.43 |
|
Lung cancer |
3.43 |
|
Asthma |
2.0–7.84,8 |
|
Steroid use |
1.6–8.03 |
|
GERD |
1.5–5.33 |
|
Rheumatoid arthritis |
1.5–1.93 |
|
Immunomodulatory/immunosuppressant therapy |
1.3–2.23 |
*Odds ratio, relative risk or relative prevalence.
Factors which increase risk of NTM-PD should be considered when deciding which patients to screen or test for infection.
Underlying lung conditions
Bronchiectasis
Bronchiectasis is known to be a risk factor for NTM-PD, but the scale of risk is variably estimated.4,9,10 NTM infection can cause bronchiectasis, and in patients with existing bronchiectasis can facilitate disease progression as the anatomically altered bronchi are susceptible to infection.11 The increased risk for developing NTM-PD in patients with bronchiectasis is estimated between 44 and 187.5-fold3,4 and the prevalence of NTM-PD among people with bronchiectasis was suggested to be 9.3%.12
Bronchiectasis is associated with NTM-PD in patients who are female and have a low-fat mass even after adjustments for BMI, age and fat mass index.13 A study in the USA has attempted to predict which patients with bronchiectasis might also have NTM-PD.14 In this study results indicated that >2 claims to a healthcare insurer within 12 months and 30 days apart of each other accurately identified pulmonary NTM infection in patients who also have bronchiectasis. However, the authors noted that the prediction had low sensitivity so that the true incidence might be severely underestimated. Importantly, a recent study11 demonstrated that NTM-PD in patients with bronchiectasis was associated with radiological changes and worsening symptoms yet despite this the Bronchiectasis Severity Score (BSI) may remain unchanged. Consequently, it is recommended that patients with bronchiectasis are tested for NTM organisms in sputum, particularly those with radiographic changes. Similarly, the ERS and British Thoracic Society (BTS) guidelines15,16 recommend that all patients with bronchiectasis being considered or receiving macrolide monotherapy should have NTM-PD ruled out.
People with bronchiectasis have an increased risk of developing NTM-PD between 44 and 187.5 fold vs those without bronchiectasis3,4
Cystic fibrosis
NTM-PD is increasing among cystic fibrosis (CF) populations worldwide. The reasons for this are unclear but may reflect the increasing lifespan of patients and the greater success that has been achieved in controlling bacterial infections like Pseudomonas aeruginosa, as well as greater awareness leading to increased NTM-PD diagnoses.7,17 Current estimates outlined in one study are that NTM infects up to 32% of CF.18 Risk of NTM-PD in people with cystic fibrosis is high with an increased prevalence of infection ranging from 6.6 to 13-fold.6,7 (Olivier 2003; Roux 2009).
A meta-analysis has demonstrated that people with CF were significantly more likely to be positive for NTM cultures if they were older (p<0.01), had Aspergillus fumigatus colonisation (OR 3.59 p<0.001), were colonised with Staphylococcus aureus (OR 1.66 p=0.001) or Stenotrophomonas maltophilia (OR 3.41 p<0.01) or were in receipt of ICS (OR 1.98 p<0.01); no other parameter showed a significant association.19 Taken together, patients with CF who also have severe conditions or infections should be closely monitored for NTM-PD.
In CF the risk of developing NTM-PD in increased, with the rates of NTM-PD in patients with CF up to 13-fold higher than in the general population,6 particularly in older patients and those with bacterial colonisation or in receipt of ICS19
COPD
COPD is a common comorbidity with NTM-PD, and a predictive modelling study20 showed that COPD was one of the highest single predictors for NTM-PD. In the USA, rates of NTM-PD in patients with COPD have been increasing since 2012,21 more than doubling between 2011 and 2015 (compared with an increase of a quarter between 2001 and 2005). Infection with NTM in patients with COPD elicited an increased mortality risk of 1.43 times that of patients with COPD and no NTM-PD,21 and a Canadian study indicated an OR for COPD as a risk factor of 15.7.4
A smaller study demonstrated that in patients with COPD, once BMI, forced expiratory volume (FEV1), and ICS use are discounted rates of NTM-PD are higher than in the general population.22 Similarly, the hazard ratio for NTM-PD in patients with pre-existing COPD was 9.15 when adjusted for sex and age, reducing only to 6.01 when fully adjusted for all factors.23 These data are important as the Canadian study by Marras et al. was a population-based study on >6 million people and the association with NTM-PD underlying COPD was studied in detail.
The overall increasing rates of COPD and the risk of NTM-PD in COPD patients indicates that changes in symptoms or radiographic changes should be investigated with NTM infection front of mind.
When other risk factors such as BMI, lung function and ICS use are discounted, COPD remains a risk factor for increased infection with NTM – up to 15.7 fold3,22,23
Asthma
Patients in receipt of inhaled corticosteroids (ICS) are known to be at increased risk of NTM infection.4,24 In one study the increase in risk of NTM-PD associated with asthma per se was 7.8.4 A nested case-control study specifically explored the association between asthma and NTM infection and suggested that patients with asthma were older, had severe airflow restriction and had been in receipt of ICS for longer (>5 years) at higher doses.8 The authors did note that receipt of ICS in these patients may be a contributing risk factor. Taken together it seems rational that asthma with its characteristic inflamed and constricted airways provides an independent risk factor for NTM-PD that is likely overlaid by additional risk because of ICS use.
Asthma increases the risk of NTM-PD up to 7.8 fold4
Immunosuppressed patients
It has long been known that using ICS increases the risk of pneumonia.25 A study in patients with asthma, COPD or COPD-asthma syndrome aged >66 years were evaluated to compare ICS use or not in those with NTM-PD. Current ICS use was associated with an increased OR of NTM-PD of 1.86 and was statistically significant for fluticasone (OR 2.09) over budesonide (OR 1.19) and the relationship between disease and ICS use was dose-dependent.24
In rheumatoid arthritis (RA) the use of biologics such as anti-tumour necrosis factor (anti-TNF) is associated with an increased risk of developing NTM-PD26 and were highest for those in receipt of adalimumab over infliximab and etanercept: 5–10 fold higher than those in RA patients not exposed to anti-TNF therapy or the general population.26,27 Similarly, in South Korea patients treated with anti-TNF a higher incidence of NTM-PD of 230 cases per 100,000 patients was reported,27 and in the USA and South Korea 70–100% of patients with RA and underlying lung disease had NTM-PD, suggesting a cumulation of risk.27 estimates the increased risk for NTM-PD in patients in receipt of immunomodulatory or immunosuppressant therapy overall at 1.3 to 2.2-fold.3
Other biologics such as rituximab (used for cancer and RA among others), abatacept, tocilizumab and ustekinumab have a theoretical increased risk of NTM-PD, but at the current time no studies are available and data is limited to small case series only.27 Similarly, in patients in receipt of immunosuppressants following transplant such as tacrolimus, NTM-PD has been identified, but again the data are limited to small case series or individual case reports.28,29
Whilst rarely seen now, an increase in NTM-PD was primarily identified in people with HIV/AIDS because of their immunosuppressed state.27 With the advent of highly active anti-retroviral therapy (HAART) there has been a sharp decline in NTM-PD cases, and it is now uncommon in people living with HIV.
For patients in receipt of immunosuppressants, particularly those with underlying lung disease, there is a need to screen those where clinical suspicion is raised. It is also vital that clinical colleagues in rheumatology or transplant medicine understand the theoretical risk for immunosuppressive therapy and NTM-PD so they can refer appropriately and promptly.
Overall, the increase in risk for NTM-PD as a result of receiving immunomodulatory or immunosuppressant therapy is 1.5–2.2 fold3
Host risk factors: patient morphology
Patient characteristics that predispose individuals to NTM-PD are well known and include:30
- Biological sex – females are at greater risk
- Thoracic skeletal abnormalities including pectus excavatum and scoliosis
- Taller than average (>165 cm for women)
- Low BMI (<20 Kg/m2)
- Leaner skinfold and limb circumference measurements than those without NTM infection.
These morphological characteristics have been initially described as those of Lady Windermere, after the character in the Oscar Wilde play Lady Windermere’s Fan,31 but it must be noted that similar morphological characteristics in males are also associated with an increased risk of NTM-PD, called Lord Windermere syndrome.32
Patient morphology can also influence disease progression once NTM-PD is diagnosed, with those with low proportions of abdominal fat at increased risk of disease progression.33 One case-controlled study indicated that tall height and a thoracic abnormality was associated with an increased odds ratio (OR) for NTM-PD of 1.1, and 5.4, respectively, whilst a BMI above normal (>26 kg/m2) was shown to be protective for NTM-PD (OR 0.11).34 Similarly, a low BMI was associated with an increased risk of NTM-PD of 9.1 fold.3 Family studies have suggested clustering of NTM-PD among related family patients with at-risk morphological characteristics suggesting an underlying genetic component.3,35
Morphological characteristics confer an increased risk of NTM-PD, tall height increases risk by 1.1-fold, thoracic abnormalities 5.4 fold and low BMI 9.1 fold3,34
Genetic predisposition
A range of genetic abnormalities are associated with an increased risk of NTM-PD and these include CF as outlined above, α1-antitrypsin deficiency (AATD) and primary ciliary dyskinesia (PCD). In both AATD and PCD, predisposition to NTM-PD is due to their impact on the lung. For AATD the appearance of COPD is very common after 30 years of age.36 In PCD genetic mutations lead to dysfunctional and uncoordinated cilial function in the lung leading to bronchiectasis; the prevalence of NTM-PD in PCD is estimated to be about 15%.37 In patients with PCD screening for NTM-PD should be done in the same way as for CF and regular sputum culture is recommended every 3–6 months.37 Similarly, in both CF and PCD training to undertake effective airway clearance is essential for patients to clear the lung to prevent infection and, if NTM-PD is present, to prevent lung damage.
Primary immune deficiency syndromes such as the rare condition Mendelian Susceptibility to Mycobacterial Disease (MSMD) is a risk factor for NTM-PD. MSMD is associated with interleukin receptor abnormalities and deformities in genes in the inflammatory cascade process.38 Likewise, a host of genetic abnormalities in the inflammatory pathway including gamma interferon (IFNγ) interleukins (IL-12) and IFN/IL receptors and macrophage proteins such as natural-resistance-associated macrophage protein 1 gene (NRAMP1) are known risk factors for NTM-PD,39 perhaps suggesting an inflammatory component to disease.
Previous or current TB
In a recent study,40 an evaluation of NTM-PD in TB suggested that one in 15 patients with TB in China also have NTM infection. The most common infecting species were M. intracellulare and M. abscessus complex. A similar co-infection state was demonstrated some years ago, with the authors suggesting that TB infection for the majority of patients follows within 6 months of confirmed NTM-PD and is significantly more common in people with a previous history of NTM-PD.41 Conversely previous TB as a pre-disposing factor to NTM-PD was suggested to confer an OR of 69.0 in the UK whilst in Denmark the risk conferred was more than doubled with an OR of 178.3.4,5
Whilst TB may be on the decline in Europe,42 it continues to be endemic in some countries, and the rise of rifampicin and multi-drug resistant TB in Europe means it remains an important disease consideration. When a patient with symptoms of NTM-PD presents and the suspicion of infection arises, patients should always be screened for previous TB.
Gastroesophageal reflux disease (GERD)
Other pre-disposing risk factors include GERD42 with an estimated increased risk of 1.5–5.3 fold.3 Patients with GERD are more likely to have acid-fast NTM bacilli and NTM-PD compared with patients without GERD and similarly, patients with MAC-PD show a high rate of GERD as a co-morbidity.42,43 The prevalence of GERD in patients with nodular NTM-PD is about 26%43 even when adjusted for other factors such as age, sex, BMI and lung function tests. Patients with GERD and NTM-PD who are in receipt of acid supressing medications are more likely to have consolidation and nodules of a size greater than 5 mm.42 It is likely that GERD is a risk factor as a result of aspiration of gut bacteria into the lung, and it is thought that acid suppression supports bacterial proliferation and survival in the gut.42
Environmental exposure
NTM are ubiquitous in the environment in soil and water supplies,43–45 and it has been considered that the environment is the reservoir for human infection. Increased NTM-PD prevalence has been linked to increased average atmospheric water vapour content,46 and the presence of NTM in home water supply is more common in patients with NTM-PD than those without disease,47 increasing the risk of NTM-PD up to 5.9-fold.3 NTM are also present in a range of other environmental and domestic scenarios but their role in infection is unclear.2 Given the pervasive nature of NTM mitigating infection is difficult, but in patients at risk simple steps such as replacing water filters regularly, avoiding hot tubs, cleaning showerheads regularly and wearing gloves when gardening can be helpful.
In summary
It is clear from a growing body of research that there exists a raft of potential risk factors that would lead a clinical suspicion of NTM-PD. But it is known that not every tall, slender woman or every patient with COPD, whilst at increased risk of NTM-PD, will develop disease, and so refining clinical suspicion is essential. These include consideration of changes or worsening in clinical condition of the patient at high risk of NTM-PD, an understanding of their medical and social history to understand iatrogenic risks in a patient’s life, and then to test and monitor patients where NTM-PD is suspected is an imperative.
Test and monitor patients with potential clinical symptoms or worsening clinical symptoms who fit the NTM-PD profile:
- Tall, slender men or women
- Patients with underlying lung conditions: asthma, bronchiectasis, COPD
- Patients with genetic conditions: CF, AATD, PCD
- Patients with GERD or environmental exposure
What to do? Rule out the presence of NTM in patients with risk factors to identify early infection and follow current treatment guidelines1 to start treatment to prevent disease progression and achieve culture conversion. Think NTM! Test NTM!
References:
1. Daley CL, et al. Eur Respir J 2020;56:2000535.
2. Cowman S, et al. Eur Respir J 2019;54:1900250.
3. Prevots DR, et al. Clin Chest Med 2015;36:13–34.
4. Andrejak C, et al. Thorax 2013;68:256–62.
5. Axson EL, et al. Eur J Clin Microbiol Infect Dis 2019;38:117–24.
6. Olivier KN et al. Am J Respir Crit Care Med 2003;167:828-34
7. Roux A-L, et al. J Clin Microbiol 2009;47:4124–28.
8. Hojo M, et al. Respirology 2012;17:185–90.
9. Shteinberg M, et al. Eur Respir J 2018; 51:1702469.
10. Ringshausen FC, et al. Emerg Infect Dis 2016;22:1102–05.
11. Chu H, et al. Arch Med Sci 2014;10:661–68.
12. Lim SY, et al. Medicine (Baltimore) 2021;100:e25193.
13. Ku JH, Diagn Microbiol Infect Dis 2020;96:114916.
14. Kwak N, et al. BMC Pulm Med 2020;20:293.
15. Smith D, et al. Thorax 2020;0:1–35.
16. Polverino E, et al. Eur Resp J 2017 ;50 :1700629.
17. Salsgiver EL, et al. Chest 2016;149:390–400.
18. Floto RA, et al. Thorax 2016;71:88–90.
19. Reynaud Q, et al. Pediatr Pulm 2020;55:2653–61.
20. Ringshausen FC, et al. Int J Infect Dis 2021;104:398–406.
21. Pyarali FF, et al. Front Med 2018;5:311.
22. Okamuri S, et al. Eur Respir J 2015;46:PA569.
23. Marras TK, et al. Eur Respir J 2016;48:928–31.
24. Brode SK, et al. Eur Respir J 2017;50:1700037.
25. Suissa S, et al. Thorax 2013;68:1029–36.
26. Winthrop KL, et al. Ann Rheum Dis 2013;72:37–42.
27. Henkle E, et al. Clin Chest Med 2015;36:91–99.
28. Suzuki H, et al. Transplant Proc 2018;50:2764–67.
29. Imoto W, et al. BMC Infect Dis 2020;20:431.
30. Kim RD, et al. Am J Respir Crit Care Med 2008;178:1066–74.
31. Reich JM, et al. Chest 1992;101:1605–09.
32. Ku JH, et al. Emerg Infect Dis 2021;27:982–85.
33. Kim SJ, et al. BMC Pulm Med 2017;17:5.
34. Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
35. Colombo RE, et al. Chest 2010;137:629–34.
36. Stoller JK, et al. 2006 Oct 27 [Updated 2020 May 21]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021. https://www.ncbi.nlm.nih.gov/books/
37. Daniels MLA, et al. Exp Opin Orphan Drugs 2015;3:31–44.
38. Ratnatunga CN, et al. Front Immunol 2020;11:303.
39. Baldwin SL, et al. PLOS Neglected Trop Dis 2019;13:e0007083.
40. Tan Y, et al. J Infect 2021;S0163-4453(21)00261-9.
41. Hsing S-C, et al. Int J Tuberc Lung Dis 2013;17:928–33.
42. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2019 Accessed July 2021
42. Thomson RM et al. Chest 2007;131:1166-72
43. Koh W-J, et al. Chest 2007;131:1825–30.
43. Lee E-S, et al. J Microbiol Biotechnol 2008;18:1207–15.
44. Falkinham JO. Clin chest Med 2015;36:35–41.
45. Falkinham JO. J Appl Microbiol 2009;107:356–67.
46. Prevots DR, et al. Annals Am Thorac Soc 2014;11:1032–38.
47. Nishiuchi Y, et al. Clin Infect Dis 2007;45:347–51.
Thank you for registering your interest
Sorry, an error has occurred: