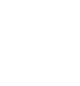When to treat?
A treatment plan should be put in place as soon as a diagnosis for NTM-PD is made
NTM treatment guidelines from 2020 recommend that patients who meet the diagnostic criteria for NTM (clinical, radiologic and microbiologic) should be treated with antibiotics immediately rather than adopt a ‘watch and wait’ approach.1,2 This is particularly true, when patients are identified to have positive acid-fast bacilli and/or lung cavitation.
However, the decision to treat patients must be individualised and based on a series of factors that affect the potential risks and benefits of therapy to the patient. These include:
- Clinical factors including the presence of lung cavities and underlying comorbidities
- Infecting species – virulence among NTM species differs: start treatment with antibiotics in those with clinically relevant species including MAC3,4
- Patient priorities and their wishes
For example, in patients with M. kansasii pulmonary disease, where aggressive therapy is recommended because of the virulence of M. kansasii, radiographic progression can be very rapid, with one study demonstrating progression within 1 year of diagnosis in 63% of patients, a 1-year mortality rate of 43% and a median survival of 71 days if patients do not receive treatment.5 By contrast, when effective treatment was initiated, progression was reduced by almost 4 fold (only 16% of patients had disease progression p<0.001).5
Similarly, in MAC-PD, in one study delays in treatment have been associated with disease progression in almost all patients (97.5%) within 6 years, with significant increases in cavitation, nodule formation and consolidation.6
It is worth remembering that for some patients at-risk of NTM-PD, they may have experienced symptoms for more than 10 years before diagnosis.7
Treating NTM-PD is a multifactorial process, and it is important to ensure that comorbidities, respiratory or otherwise, are also optimally managed and address issues such as weight loss, diet and nutrition, depression and sleep disorders.
NTM-PD treatment decisions are often difficult and require experience in managing the disease, consider referral to an expert centre or consultation with experienced peers.1



How to treat?
Treating NTM-PD from non-pharmacological considerations to surgery
Treating NTM-PD effectively should consider all available options including non-pharmacological airway clearance, through to antibiotic therapy and surgery.1,8,9
Patients often face a long journey to diagnosis, and treatment for NTM-PD can be extremely challenging to patients with lengthy and potentially complicated dosing regimens that may be associated with adverse events and poor patient adherence to therapy.10
Patients with NTM-PD are known to have a deteriorating health-related quality of life (HRQoL) as a result of their disease and treatment options, therefore setting their expectations at the outset of treatment is critical to success.11
Airway clearance is vital at all stages of NTM-PD management
Airway clearance is an important aspect of treatment for all patients with NTM-PD helping to expel mucus from the lungs.
It can improve the volume of sputum removed from the lungs, cough symptoms, breathlessness and HRQoL.8
Techniques for airway clearance include:
- Nebulised hypertonic saline
- Manual chest physical therapy
- Device led clearance such as oscillating devices and inflatable vests
- Cough techniques – huff coughing
Antibiotic management of NTM-PD
Guidelines published in 2020 provide multidrug management strategies for patients infected with NTM species, specifically Mycobacterium avium complex (MAC), M. kansasii, M. xenopi and M abscessus.1
Surgery for the treatment of NTM-PD
NTM-PD if often difficult to treat with antimicrobial therapy alone. For selected patients, surgical resection may be appropriate and should be considered alongside pharmacological therapy.1,9
The decision to undertake surgical resection requires expert consultation but studies suggest that culture conversion following surgery is comparable to that seen with antimicrobial therapy.1
Regardless of the treatment options chosen, active monitoring of patients including regular sputum sample analysis and clinical follow ups is essential.
Susceptibility testing
Understanding the susceptibility of NTM to key antibiotics is essential for successful therapy
Once a decision to treat NTM-PD is made, it is recommended to establish susceptibility of the organism to the first-line antibiotic.1 Tailored susceptibility-led treatment is preferred and recommended over empiric therapy.1,12
Susceptibility testing is not able to predict which patients with NTM-PD will respond to antibiotic therapy but is important to inform therapeutic decisions and should always be undertaken in patients failing to respond to therapy.1,12
The guidelines recommend the following susceptibility testing, depending on the infecting organism identified
- MAC-PD: macrolides (azithromycin and clarithromycin) and amikacin
- M. kansasii: rifampicin
- M. abscessus: macrolides and amikacin, and for macrolides evaluate potential inducible macrolide resistance through 14-day incubation and/or sequencing of the erm(41) gene
- M. xenopi: at the present time there is insufficient evidence to be able to recommend specific susceptibility testing
In cases of macrolide resistance, IV amikacin or streptomycin are added to the regimen.1 Similarly, susceptibility of amikacin is important to establish, as patients with severe or fibrocavitary disease should receive IV amikacin or streptomycin plus standard antibiotic triple-therapy of macrolides, preferably azithromycin, ethambutol and rifampicin, for the first 2–3 months of treatment.
For those patients for whom oral guideline-based triple-therapy fails after 6 months, amikacin liposomal inhalation suspension (ALIS) is recommended, and the breakpoint for ALIS is much higher than that for IV amikacin (≥ 128 mg/L vs ≥64 mg/L).1,13,14
Most recent guidelines for macrolide use in bronchiectasis, a common comorbidity for MAC-PD, recommend all patients being considered for macrolide therapy, to treat or mitigate exacerbations, be screened for macrolide susceptibility and the presence of NTM, in order to preserve macrolides for treatment of NTM if identified.15
Treatment guidelines
The journey of treating NTM-PD focusses on early treatment with oral guideline-based therapy
The decision to treat is influenced by:
- identity of infecting mycobacterium
- severity of NTM-PD
- risk of disease progression
- presence of comorbidity
- goals of treatment
Whilst the reasons are not understood the best chance of treatment success for NTM infection such as MAC-PD is with the first course of guideline-based antimicrobial therapy.16 Establishing the right course of treatment for your patient is essential to improve the chance of treatment success.
Guidelines provide treatment recommendations for those mycobacterial species that are most common and associated with highest pathogenicity – MAC, M. kansasii¸ M. xenopi and M. abscessus.1
Treatment guidelines provide an overview of treatment approaches for susceptible and resistant organisms. In the case of MAC-PD, the guidelines also provide treatment recommendations for patients who fail to culture convert at 6 months.1
In patients where M. kansasii is identified, treatment should be initiated after a single positive culture because of its pathogenicity.1 Patients with M. kansasii are at a high risk of mortality within 1 year after diagnosis if not treated.5 In these patients, M. kansasii should be treated with a recommended regimen of rifampicin, ethambutol and either a macrolide or isoniazid and may be administered intermittently in those with non-cavitary nodular disease. For patients with rifampicin resistance or an intolerance to a drug in first-line treatment, consider adding a fluoroquinolone such as moxifloxacin.1
In patients with MAC-PD, once susceptibility has been established, guidelines recommend the use of at least three oral antibiotics of a macrolide (azithromycin preferred over clarithromycin), ethambutol and rifampicin, for the initial treatment of MAC-PD, with the addition of parenteral aminoglycosides for those with macrolide-resistant infection.1
For patients with MAC-PD in whom oral GBT has failed after ≥6 months, for patients with amikacin susceptible isolates guidelines recommend adding ALIS14 to the dosing regimen.1 The breakpoint for ALIS is ≥128 μg/mL compared with IV amikacin which is ≥64 μg/mL.13
In patients with M. abscessus without inducible resistance then a macrolide containing multidrug regimen is recommended. Similarly, in those with inducible macrolide resistance continue to include a macrolide for its immunomodulatory properties only alongside a regimen of other active antimicrobials.1 M. abscessus is associated with high rates of mortality and antimicrobial resistance.17,18
For patients infected with M. xenopi treatment should be initiated with a multidrug regimen of at least 3 antimicrobials that includes moxifloxacin or a macrolide plus rifampicin and ethambutol.1 As M. xenopi is associated with a high rate of mortality at least a 3-drug regimen should be used, and parenteral amikacin added to the regimen in those with cavitary disease.1
Data indicate that oral GBT, in patients with NTM-PD excluding MAC, fails in 34% of patients8 and can be high as 45% in those with MAC-PD.20–22
Once treatment is underway it is important to closely monitor patients to evaluate the point of culture conversion. Successful treatment of NTM-PD requires:1,2
- Monitoring through evaluation of frequent sputum cultures
- Clinical improvement within 3–6 months
- Culture conversion within 6 months of appropriate antibiotic therapy
- Sustained culture conversion
Once culture conversion occurs, guidelines recommend continuing treatment for a further minimum 12 months beyond culture conversion for those infected with MAC, M. kansasii and M. xenopi and for M. abscessus it is recommended to seek expert advice to determine treatment duration.12
Ongoing disease management
 Monitoring plus treatment for 12 months beyond the point of culture conversion is key to successful ongoing NTM-PD management
Monitoring plus treatment for 12 months beyond the point of culture conversion is key to successful ongoing NTM-PD management
Proactive management is key to treatment success in NTM-PD. Monitoring and supporting patients through the course of treatment needs to focus on clinical and patient aspects of the disease.
To track patient progress, sputum cultures should be evaluated regularly every 1–2 months, and once patients culture convert, treatment should continue for a further 12 months.1,23 During this time, serial sputum cultures should continue with a frequency determined by clinical need but may be as frequent as every 4-12 weeks.24 Monitoring the course of disease and treatment success relies on a combination of clinical, microbiological, and radiological parameters in order to evaluate symptoms, sputum as well as lung function and structural damage.1,25
Alongside NTM-PD specific treatment, patients should be evaluated holistically. It is known that the use of immunosuppressive medications such as inhaled corticosteroids or biological agents is a risk factor for NTM, as are patient predisposition factors such as low BMI, female sex, vitamin D deficiency and underlying diseases such as bronchiectasis, COPD, GORD, diabetes and chronic kidney disease.25 Some of these elements are modifiable and should be addressed through a multidisciplinary team approach.25
Within the multidisciplinary model, the respiratory physician, radiologist and microbiologist are essential. However, as a chronic disease with a treatment course of more than a year, it has been suggested that a multidisciplinary team for NTM should include a specialist nurse role as patients require ongoing support.25 Effective patient support may improve treatment adherence through both clinic visits and virtual contact via telephone or online consultations.
Countering progressive decline in lung function is a key component of care in NTM-PD and throughout the disease course, patients need to be supported with airway clearance, most often through the help of a physiotherapist.25 Similarly, as NTM-PD is linked to low BMI25,26 establishing improvements in nutrition are important. The impact of disease on a patient’s quality of life should not be underestimated so psychological interventions should be considered for individual patients as well as support from local/national patient groups.25
For patients undergoing initial antimicrobial therapy with oral GBT, studies have shown that treatment success with oral GBT varies by NTM species from 32% to 80% (32% M. xenopi, 41%, MABC, 66% for MAC, 80% M. kansasii).27,28
Featured Content

Video
Duration: 5 mins
In this video European experts provide their insights into the 2020 ATS/ERS/ESCMID/IDSA guidelines on NTM-PD, with a focus on MAC-PD.

Article
Read time: 5 mins
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) with antimicrobial agents offers the possibility of cure. In patients who meet the clinical, radiographical and microbiological diagnostic criteria for NTM-PD.

Article
Read time: 7 mins
Mycobacterium avium complex pulmonary disease (MAC-PD) is difficult to diagnose with symptoms similar to underlying lung conditions. Correct, early diagnosis and treatment are paramount to prevent disease progression.

Video
MAC: What to do in the event of MAC pulmonary disease (MAC-PD) treatment failure?
Duration: 4 mins
In a substantial number of patients with MAC-PD, first-line treatment with guideline-recommended triple-therapy will not provide culture conversion. In this video experts explore what options clinicians have in MAC-PD when treatment fails.

Video
MAC: Initiation of treatment of MAC pulmonary disease (MAC-PD)
Duration: 15 mins
Knowing when to initiate treatment for MAC-PD is a multifactorial decision. In this video experts explore the rationale and timing for starting treatment.

Video
MAC: Ongoing management of MAC pulmonary disease (MAC-PD) patients
Duration: 9 mins
Once treatment is initiated, monitoring patients for a response is vital in order to plan next steps. See and hear international experts explore the key elements of ongoing treatment up to and beyond culture conversion.

Video
NTM: 2020 ATS, ERS, ESCMID, IDSA and NTM-PD Guidelines — an expert overview
Duration: 33 mins
Stefano Aliberti, Christoph Lange, Eva Polverino, Nicolas Veziris, Charles Haworth and Jakko van Ingen
Non-tuberculous mycobacterial pulmonary disease (NTM-PD) can be life threatening and is increasing in prevalence. International guidelines updated in 2020 provide management recommendations for the four most commonly occurring NTM pathogenic species.

Article
Read time: 5 mins
The CONVERT study [NCT02344004] evaluated the efficacy and safety of amikacin liposomal inhalation suspension (ALIS) in patients with treatment-refractory NTM-PD
Find out more about NTM-PD
Test your NTM knowledge
Get involved and test your knowledge of the 2020 guidelines with questions based on clinical scenarios
References:
- Daley CL et al. Eur Respir J 2020;56:2000535.
- Griffith DE et al. Am J Respir Crit Care Med 2007;175:367–416.
- Fleschner M et al. Int J Tuberc Lung Dis 2016;20:582–7.
- Zweijpfenning S et al. Semin Respir Crit Care Med 2018;39:336–42.
- Liu C-J et al. Respir Med 2019;151:19–26.
- Park TY et al. PLoS One 2017;12:e0185774.
- Kotilainen H et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
- Lan C-C et al. J Formosan Med Assoc 2020;119:S42–S50.
- Yu JA et al. Thorac Surg Clin2012;22(3):277–85.
- Chalmers et al. ERJ Open Research 2020;6: 00317-2020
- Henkle E et al. Eur Respir J 2020;55:1901300.
- Haworth C et al. Thorax 2017;72(Suppl 2):ii1–ii64.
- Clinical and Laboratory Standards Institute. M48 - laboratory detection and identification of mycobacteria, 2nd edition 2018.
- ARIKAYCE liposomal. European Summary of Product Characteristics June 2023. Available at: https://www.ema.europa.eu/en/documents/product-information/arikayce-liposomal-product-information_en.pdf [Accessed January 2024]
- Smith D et al. BMJ Open Respiratory Res 2020;7:e000489.
- Griffith DE et al. Curr Opin Infect Dis 2012;25:218–27.
- Jhun BW et al. Eur Respir J 2020;55:1900798.
- Sfeir M et al. Open Forum Infect Dis 2018;5(2):ofy022.
- Aliberti S et al. Respir Med 2020 Absract.
- Fukushima J et al. J Clin Med 2020;9:1315.
- Wallace RJ et al. Chest 2014;146:276–82.
- Diel R et al. Chest 2018;153:888–921
- Daley CL. Microbio Spectr 2017;5:1–36.
- Whitehead N. Nursing Times 2021;117;4:40–42.
- Lipman M et al. BMJ Open Resp Res 2020;7:e000591.
- Prevots DR et al. Clin Chest Med 2015;36:13–34.
- Diel R et al. Eur Resp J 2017;49:1602109.
- Diel R et al. BMC Infect Dis 2018;18:206.
Benefits of early treatment initiation in non-tuberculous mycobacterial pulmonary disease (NTM-PD)
Treatment of non-tuberculous mycobacterial pulmonary disease (NTM-PD) with antimicrobial agents offers the possibility of cure.1 In patients who meet the clinical, radiographical and microbiological diagnostic criteria for NTM-PD, the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for NTM-PD recommend initiation of treatment rather than watchful waiting.1 Initiation is especially important in the context of positive acid-fast bacilli sputum smears and/or cavitary lung disease1as there may be an increased rate of progression and poor treatment outcomes if treatment is delayed.1
Evidence of disease progression in untreated MAC-PD
Several studies have shown that most patients diagnosed with Mycobacterium avium complex pulmonary disease (MAC-PD) have progressive disease resulting in the need for antibiotic treatment.2,3 In a recent study of 488 newly diagnosed patients at the Asan Medical Center in South Korea, 305 (62.5%) patients showed progressive MAC-PD resulting in treatment initiation within 3 years of diagnosis.2 Similarly, in another study of 40 untreated patients with the nodular bronchiectatic form of MAC-PD (most with minimal symptoms), who underwent serial chest computed tomography (CT) scans for a minimum of 4 years, 39 (97.5%) experienced disease progression with a significant increase in overall CT score.3 It is noted in the 2020 NTM-PD guidelines that some subgroups (minimal nodular/bronchiectatic disease) may be safely, but regularly, followed without antimicrobial therapy; however, those with cavity disease should always receive prompt antibiotic treatment.1
Factors influencing the decision to initiate treatment
The decision to treat may be influenced by both host factors and infecting bacterial species. Certain factors like cavitary disease and low body mass index have been associated with progressive disease and may necessitate earlier consideration of antibiotic treatment.2 In very frail patients with very mild nodular bronchiectatic disease, the balance between efficacy and tolerability may favour watchful waiting.1
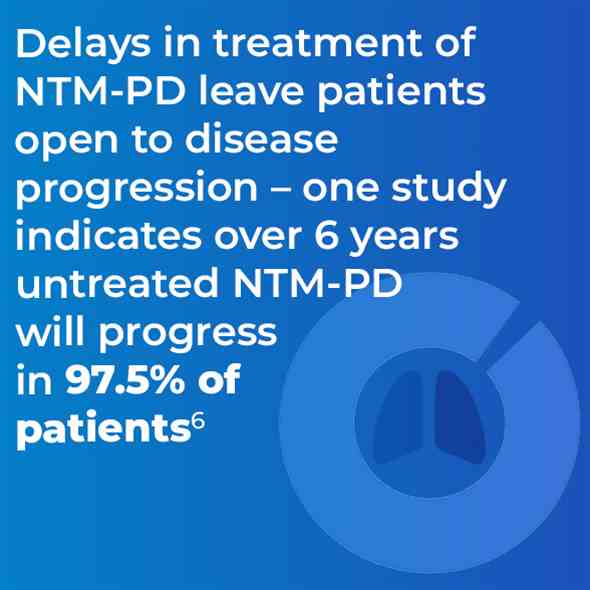
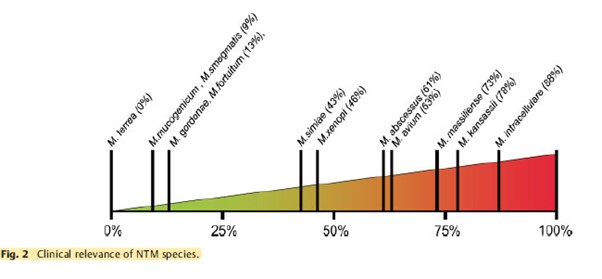
The clinical relevance of NTM varies significantly between species (Figure 1) and may also differ geographically.1,4 For example, species such as M. gordonae have low pathogenicity and rarely cause disease in humans, whereas M. kansasii is highly pathogenic.1,4
Figure 1. Clinical relevance (the percentage of patients with isolates of these species that meet the ATS/IDSA diagnostic criteria) of non-tuberculous mycobacterial species. M., Mycobacterium. Adapted from Zweijpfenning (2018).5
The most common NTM pathogens include MAC, M. kansasii and M. xenopi among the slowly growing NTM and M. abscessus among the rapidly growing NTM.1
Meeting the guideline-recommended diagnostic criteria for NTM-PD
Diagnostic criteria within the guideline is based on:
- Clinical symptoms e.g. worsening of symptoms of underlying lung conditions, or onset of new, persistent symptoms in patients at risk of NTM-PD e.g. haemoptysis, weight loss, fatigue
- Radiological findings on X-ray or hight-resolution CT scan such as nodular or cavitary opacities
- Microbiological findings from a) at least two expectorated sputum or b) positive culture from at least one bronchial wash or lavage or c) positive culture for NTM and biopsy from transbronchial or lung biopsy plus one or more culture positive sputa or bronchial washing.
Patients suspected of having NTM-PD who do not meet the diagnostic criteria should be actively managed and followed with serial CT scans until the diagnosis is firmly established or excluded and should start or continue recommended techniques such as airway clearance.6
The decision to initiate antibiotic treatment
NTM-PD is associated with diminished health-related quality of life that correlates with severity of lung impairment;7 antimicrobial treatment may be associated with improvement.8
NTM-PD treatment decisions are often difficult and require experience in managing the disease. This can mean that it may be necessary for a peer consultation or referral to a pulmonologist or infectious disease specialist with experience in NTM-PD.1,9 The virulence and potential for progressive disease must be evaluated once the NTM species is identified in order to determine treatment. In the 2020 ATS/ERS/ESCMID/IDSA clinical practice guideline for NTM-PD for example, it is recommended that for species of low pathogenicity such as M. gordonae, treatment is only indicated if repeated positive cultures over several months are observed, along with strong clinical and radiological evidence of disease whereas in many patients only one positive M. kansasii sputum culture may be required in order to initiate treatment.1 Similarly, clinically significant MAC-PD is unlikely in patients who have a single positive sputum culture during the initial evaluation but can be as high as 98% in those with ≥2 positive cultures.1 Two or more MAC-positive cultures indicate active MAC infection requiring a treatment decision, whereas for patients identified with M. kansasii, treatment should be initiated as soon as a single positive culture is obtained.1
Regardless of the infecting organism, the decision to initiate antibiotic treatment should be individualised considering the patient’s symptoms, the pathogenicity of the organism, radiological findings, microbiological results and importantly, the patient’s wish and ability to receive treatment as well as the goals of therapy.1 Any treatment decision should include a discussion with the patient that outlines the potential side-effects of antimicrobial therapy, the uncertainties surrounding the benefits of antimicrobial therapy and the potential for recurrence including reinfection (particularly in the setting of nodular/bronchiectatic disease).1 Guidelines recommend regular sputum cultures and routine monitoring to assess disease progression.1
 Following treatment initiation, sputum specimens should be obtained for culture every 1 to 2 months to document when sputum cultures become negative and to survey for the appearance of other organisms. 1
Following treatment initiation, sputum specimens should be obtained for culture every 1 to 2 months to document when sputum cultures become negative and to survey for the appearance of other organisms. 1
Clinical and radiographical assessments should be performed alongside the microbiological assessments to determine if the patient is responding to therapy.1
Retrospective studies have shown that most patients with MAC-PD who convert on treatment do so within 6 months of starting treatment.11–13
If you decide not to initiate antibiotic treatment, an active monitoring plan is recommended by the guidelines.1 Study data suggest that untreated NTM-PD could progress.2,3
References:
- Daley Cl, et al. Clin Infect Dis 2020;71:e1–e36.
- Hwang JA, et al. Eur Respir J 2017;49:1600537.
- Park TY, et al. PLoS One 2017;12:e0185774.
- van Ingen J, et al. Thorax 2009;64:502–6.
- Zweijpfenning SMH, et al. Semin Respir Crit Care Med 2018;39:336–42.
- Lipman M, et al. BMJ Open Respir Res 2020 ;7 :e000591
- Mehta M, Marras TK. Respir Med 2011;105:1718–25.
- Czaja CA, et al. Ann Am Thorac Soc 2016;13:40–8.
- Ryu YJ, et al. Tuberc Respir Dis 2016;79:74–84.
- Lee MR, et al. Clin Microbiol Infect 2015;21:250.e1–250.e7.
- Furuuchi K, et al. Chest 2020;157:1442–5.
- Koh WJ, et al. Eur Respir J 2017;50:1602503.
- Moon SM, et al. Eur Respir J 2019;53;1801636.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
,
,
An overview of the rationale and approach to diagnosis of Mycobacterium avium complex pulmonary disease (MAC-PD)
Mycobacterium avium complex pulmonary disease (MAC-PD) is difficult to diagnose with symptoms similar to underlying lung conditions.1 Correct, early diagnosis and treatment are paramount to prevent disease progression.1–4 The 2020 international guidelines recommend clinical, radiographical and microbiological diagnostic criteria for non-tuberculous mycobacterial pulmonary disease (NTM-PD) to facilitate timely and appropriate treatment.5
NTM-PD: an overlooked disease caused by ubiquitous mycobacteria
Non-tuberculous mycobacteria pulmonary disease (NTM-PD) is a chronic and potentially debilitating disease.6–10 Mycobacteria are ubiquitous in the environment11–13 and comprise almost 200 species and subspecies that can cause opportunistic infections in both pulmonary and extrapulmonary sites.5 NTM infections can be difficult to diagnose and treat and are particularly prevalent in those with lung damage or disease, cancer and immunodeficiencies.14
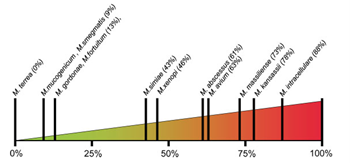
A series of 12 molecularly related Mycobacterium species have been identified that together comprise the Mycobacterium avium complex (MAC).15 Within MAC, the two most clinically relevant species are M. avium and M. intracellulare.16,17 MAC is the most common cause of NTM.18
Figure 1. Worldwide distribution of respiratory NTM isolates.
(Adapted from Hoefsloot, 2013)18
Increasing prevalence of NTM-PD
The incidence and prevalence of NTM-PD is increasing in many parts of the world.1,5,19,20 This may reflect increased awareness of the importance of NTM,1 the incidence of risk factors such as chronic obstructive pulmonary disease (COPD) and bronchiectasis,21,22 the use of immunosuppressive treatments,23 testing for NTM-PD and the effectiveness of diagnostic tools.1,17,24 NTM should be ruled out in at-risk patients to identify infection early and begin treatment in appropriate patients before there is disease progression.
Early diagnosis of NTM-PD is paramount to prevent disease progression.
NTM-PD is associated with increased mortality and morbidity – increasing the risk of pulmonary exacerbations9, lung cancer25 and other lung infections26 (e.g. tuberculosis [TB], aspergillosis) and atrial fibrillation.27 NTM-PD is difficult to diagnose as the symptoms of NTM-PD – cough, fatigue, haemoptysis, weight loss – are similar to underlying lung conditions.1 Many patients with NTM-PD may experience symptoms for >10 years before diagnosis.28 Correct, early diagnosis and treatment are paramount to prevent disease progression.1–4 One study has shown that without treatment, 97.5% (n=39/40) of MAC-PD patients will have disease progression within 6 years.17 Another study showed that for those with M. kansasii progression can be very rapid in untreated patients within 1 year (progression in up to 63% of patients) and a median survival of 71 days.29 Late diagnosis, misdiagnosis or inappropriate management of NTM-PD is likely to increase the risk of deterioration in lung health and health-related quality of life.
NTM-PD – not all species are equal
Not all NTM species will cause disease, and when they do, they may not need to be treated.1,30 Likewise the geographical distribution of species differs, driving local epidemiology. Knowledge of the local situation and species virulence is essential for daily clinical practice. MAC is most frequently associated with NTM-PD across all continents with M. avium causing about 63% of infections meeting ATS/IDSA criteria for treatment and M. intracellulare about 88%. Species such as M. gordonae are rarely clinically relevant.30
Who is at risk of NTM-PD?
For many at-risk patients NTM-PD symptoms are similar to symptoms of coexisting lung disease.1 These include chronic cough, fatigue, weight loss and low-grade fever.
Patients most at risk of developing NTM-PD include those with:
- Lung diseases e.g. bronchiectasis, COPD, asthma, prior TB.31–34
- Diseases/disorders causing structural lung damage e.g. cystic fibrosis, rheumatoid arthritis, genetic mutation.35–38
- Thoracic skeletal abnormalities – scoliosis, kyphosis, pectus excavatum:39,40 five times more likely to have NTM.34
- Diseases/disorders reducing cell-mediated immunity e.g. HIV AIDS, cancer, genetic mutation.37,38,41
- Therapies resulting in immunodeficiency e.g. organ transplant, anti-tumour necrosis factor (TNF) therapy, corticosteroids, immunosuppressants.34–36,41–44
- Marfanoid body habitus/Lady Windermere syndrome39,40,45 – tall, slender elderly patients with a below normal body mass index (BMI) (<18 Kg/m2) are three times more like to have NTM.34
|
Factors increasing susceptibility to NTM-PD |
Risk* |
|
Bronchiectasis32,47 |
44.0–187.5 |
|
Low BMI47 |
9.1 |
|
Cystic fibrosis65,66 |
6.6–13.0 |
|
COPD47 |
2.0–10.0 |
|
Thoracic skeletal abnormalities47 |
5.4 |
|
Asthma67 |
2.0 |
|
Steroid use47 |
1.6–8.0 |
|
GORD47 |
1.5–5.3 |
|
Immunomodulatory/immunosuppressant therapies47 |
1.3–2.2 |
* Relative risk, odds ratio or relative prevalence
Rates of NTM-PD are high in older individuals and those with underlying bronchiectasis.46,47 Many patients with bronchiectasis also have COPD (36–51%) or asthma (28–42%).23
In which patients should I rule out NTM?
NTM-PD should be ruled out in patients with underlying structural lung disease who:
- are being considered for long-term macrolide therapy to reduce exacerbations48
- are already receiving long-term macrolide therapy48
- present with worsening symptoms despite treatment optimisation49
- present with new pulmonary and non-specific systemic symptoms (e.g. chronic cough, fatigue, fever or dyspnoea).49
MAC-PD: difficult to treat
MAC-PD can be difficult to treat; MAC organisms evade host defences; they accumulate in biofilms and their uptake in macrophages gives them a place to hide from many antibiotics, which have poor penetration of these cells.50–54 Once inside macrophages, MAC limits normal macrophage function and reproduces unhindered, ready to trigger macrophage destruction so MAC bacteria can be released to infect the lung and invade other macrophages.55–57
Diagnostic criteria for MAC-PD
The 2020 international guidelines recommend that MAC-PD is diagnosed with either X-ray or computed tomography (CT) scan and the presence of MAC-positive sputum on multiple occasions. For radiological evidence of MAC-PD, either nodular or cavitary opacities on a chest radiograph, or bronchiectasis with multiple small nodules on a high-resolution CT scan is required. For microbiological confirmation, the 2020 guidelines recommend:5,19
- >1 positive sputum culture (to avoid spurious results from environmental contamination) with >3 respiratory samples collected over 1 week (to distinguish MAC-PD from occasional presence of MAC in the tracheobronchial tract).
- If sputum specimens are not obtainable, bronchoalveolar lavage fluid/bronchial washing cultures can be used to diagnose nodular/bronchiectatic NTM disease.
- Transbronchial or other lung biopsy with mycobacterial histologic features e.g., granulomatous inflammation or acid-fast bacilli (AFB) and positive culture for NTM or biopsy and one or more culture positive sputum or bronchial washings
Species identification helps determine clinical relevance and treatment selection.1,30 Where the same species is isolated in ≥2 sputum cultures over an interval of ≥1 week, there is a 98% likelihood of clinically significant MAC.
Following confirmation of a MAC-PD infection and the decision to treat, the next step is to test for antibiotic susceptibility5 to facilitate the selection of appropriate antimicrobial therapy.
Decision to treat MAC-PD
The decision to initiate antibiotic therapy for MAC-PD should not be based on diagnostic criteria alone.5,19 It is influenced by the severity of the disease, risk of disease progression, species/pathogenicity of infecting Mycobacterium, drug susceptibility, presence of comorbidity and the goals of treatment.1,5,19
Factors favouring treatment include those associated with poor prognosis (e.g. cavitary disease, low BMI, low albumin and elevated inflammatory markers), isolation of a species that is virulent and/or responsive to antimicrobial therapy, underlying immune suppression and major symptoms causing decreased health-related quality of life (e.g. fatigue).5,19 International NTM management guidelines recommend early treatment, as the benefits may outweigh the risks.5 Patients with NTM may already have a high treatment burden from their underlying chronic condition58–60 which could lead to a reluctance to add to it by starting treatment for NTM-PD immediately. However, with evidence from a study which suggests that left untreated, MAC-PD will progress,17 and increase risk of all-cause mortality27,61 and morbidity6–9,62 lowering patient quality of life,26,63 prompt treatment is essential.
Summary
Understanding which patients with underlying lung conditions are at risk of developing MAC-PD is important in order to deliver prompt and effective therapy. Treatment for MAC-PD is most successful at first initiation1 and a study has shown that refractory disease is associated with use of non-standard treatment regimens as outlined in the guidelines.64
Understanding the need to identify at-risk patients, implement effective diagnostic protocols and prompt robust therapy is a medical imperative with the potential to reduce patient mortality and morbidity.
References:
- Griffith DE, et al. Am J Respir Crit Care Med 2007;175:367–416.
- Eikhani MS, et al. BMC Infect Dis 2018;18:311.
- Maiga M, et al. PLoS One 2012;7:e36902.
- Wagner D, et al. Poster presented at: European Respiratory Society Annual Congress 6–10 September 2014; Munich, Germany. P1067.
- Daley CL, et al. Eur Respir J 2020b;56(1):2000535.
- Park HY, et al. Chest 2016;150:1222–32.
- Kobayashi T, et al. J Clin Tuberc Other Mycobact Dis 2018;11:17–21.
- Lee MR, et al. PLoS One 2013;8:e58214.
- Huang CT, et al. Int J Tuberc Lung Dis 2012;16:539–45.
- Marras TK, et al. Emerg Infect Dis 2017;23:468–76.
- Falkinham JO. J Appl Microbiol 2009;107:356–671.
- Falkinham JO. Clin Chest Med 2015;36:35–41.
- Nishiuchi Y, et al. Front Med 2017;4:27.
- Ratnatunga CN, et al. Front Immunol 2020;11:303.
- van Ingen J, et al. Int J Syst Evol Microbiol 2018;68:3666–77.
- Boyle DP, et al. Am J Respir Crit Care Med 2015;191:1310–17.
- Park TY, et al. PLoS One 2017;12:e0185774.
- Hoefsloot W, et al. Eur Respir J 2013;42:1604–13.
- Daley CL, et al. Clin Infect Dis 2020a;71:e1–e36.
- Diel R, et al. BMC Infect Dis 2018;18:206.
- Terzikhan N, et al. Eur J Epidemiol 2016;31:785–92.
- Snell N, et al. Respir Med 2019;158:212–3.
- Chalmers JD, et al. Pulmonol 2018;24:120–31.
- Chalmers JD, et al. Chest 2018;165:1272–3.
- Taira N, et al. Am J Case Rep 2018;19:748–51.
- Yeung MY, et al. Respirology 2016;21:1015–25.
- Park CS, et al. Sci Rep 2019;9:15503.
- Kotilainen H, et al. Eur J Clin Microbiol Infect Dis 2015;34:1909–18.
- Liu C-J, et al. Respir Med 2019;151:19–26.
- Zweijpfenning SM, et al. Semin Respir Crit Care Med 2018;39:336–42.
- Aksamit TR, et al. Chest 2017;151:982–92.
- Andrejak C, et al. Thorax 2013;68:256–62.
- Jones MM, et al. PLoS One 2018;13:0197976.
- Dirac MA, et al. Am J Respir Crit Care Med 2012;186:684–91.
- Winthrop KL, et al. Ann Rheum Dis 2013;72:37–42.
- Brode SK, et al. Thorax 2015;70:677–82.
- Wu UI, et al. Lancet Infect Dis 2015;15:968–80.
- Szymanski EP, et al. Am J Respir Crit Care Med 2015;192:618–28.
- Kim RD, et al. Am J Respir Crit Care Med 2008;178:1066–74.
- Holt MR, et al. Eur Respir J 2019;54:1900252.
- Henkle E, et al. Clin Chest Med 2015;36:91–9.
- Ose N, et al. Surg Case Rep 2019;5:11.
- Friedman DZP, et al. Transpl Infect Dis 2020;22:e13229.
- Chao WC, et al. BMC Infect Dis 2017;17:796.
- Ku JH, et al. Diagn Microbiol Infect Dis 2020;96:114916.
- Prevots DR, et al. Am J Respir Crit Care Med 2010;182:970–6.
- Prevots DR, et al. Clin Chest Med 2015;36:13–34.
- Smith D, et al. BMJ Open 2020;7:e000489.
- Haworth C, et al. Thorax 2017;72:ii1–ii64.
- Awuh JA, et al. Cell Mol Life Sci 2017;74:1625–48.
- Ganbat D, et al. BMC Pulm Med 2016;16:19.
- Esteban J, et al. Front Microbiol 2018;8:2651.
- Chakraborty P, et al. Microbiol Cell 2019;6:105–22.
- McGarvey, et al. Clin Chest Med 2002;23:569–83.
- Chiplunkar SS, et al. Future Microbiol 2019;14:293–313.
- Gomes MS, et al. Infect Immun 1999;67:3199–206.
- Lee K-I, et al. Sci Rep 2016;6:37804.
- Global Burden of Disease 2015 Chronic Respiratory Disease Collaborators. Lancet Respir Med 2017;5:691–706.
- Lopez-Campos J, et al. Respirology 2016;21:14–23.
- Redondo M, et al. Breathe 2016;12:222–35.
- Diel R, et al. Eur Resp J 2017;49:1602109.
- Fleshner M, et al. Int J Tuberc Lung Dis 2016;20:582–7.
- Mehta M, et al. Respir Med 2011;105:1718–25.
- Fukushima K, et al. J Clin Med 2020;9:1315.
- Olivier KN, et al. Am J Respir Crit Care Med 2003;167:828–34.
- Roux A-L, et al. J Clin Microbiol 2009;47:4124–8.
- Hojo M, et al. Respirology 2012;17:185–90.
![]() Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
The efficacy, sustainability and long-term safety of ALIS for patients with treatment-refractory MAC-PD
The CONVERT study [NCT02344004] evaluated the efficacy and safety of amikacin liposomal inhalation suspension (ALIS) in adult patients with treatment-refractory non-tuberculous mycobacterial pulmonary disease (NTM-PD) caused by Mycobacterium avium complex (MAC) in addition to oral guideline-based therapy (GBT) compared with oral GBT alone. ALIS plus oral GBT demonstrated high rates of culture conversion in 6-month data published in 2018 compared with GBT alone (29% vs 9%) and a follow-up study demonstrated culture conversions were often sustained and durable, and there were no new safety signals emerged with long-term use of ALIS.
MAC-PD is a difficult-to-treat pulmonary infection. When initial oral GBT fails, outcomes are poor and options are limited.1,2 ALIS is a novel amikacin formulation that penetrates alveolar macrophages and biofilms while limiting systemic exposure.3–5 ALIS is currently recommended by guidelines for patients with MAC-PD who fail to achieve culture conversion after at least 6 months of oral GBT in combination with oral GBT.6 ALIS has been previously tested in a Phase II study of treatment-refractory non-tuberculous mycobacterial pulmonary disease (NTM-PD) where the addition of ALIS to standard oral GBT achieved higher rates on negative sputum cultures compared with oral GBT alone.7
CONVERT was a prospective, open-label, randomised trial that evaluated the efficacy and safety of daily ALIS in addition to oral GBT in patients with refractory MAC-PD compared with oral GBT alone. A total of 336 patients with amikacin-susceptible MAC-PD and MAC-positive sputum cultures after receiving at least 6 months of oral GBT were randomised at a 2:1 ratio to receive either ALIS plus oral GBT or oral GBT alone. The primary endpoint was the proportion of patients achieving culture conversion, which was achieved if patients had three consecutive monthly MAC-negative sputum cultures by Month 6 of the study. The study was conducted in 127 centres across 18 countries in North America, Asia-Pacific and Europe. Patients were mostly female (69.3%) with a mean age of 64.7 years and many patients had underlying bronchiectasis (62.5%), chronic obstructive pulmonary disease (14.3%) or both (11.9%). The majority of patients (89.9%) were receiving GBT at enrolment, with the remainder off treatment for 3–12 months. Most patients (69.3%) were on a three-drug regimen at baseline, with 54.9% on regimens which included a macrolide, ethambutol and a rifamycin.8
Initial results of the trial demonstrated the efficacy of ALIS in addition to oral GBT in achieving culture conversion by Month 6.8 Patients treated with ALIS in addition to oral GBT were almost four times as likely to achieved culture conversion by Month 6,8 with 29% (n=65/224) of patients on ALIS plus oral GBT achieving culture conversion compared with only 8.9% (n=10/112) on oral GBT alone (P<0.0001).8,9
In a follow-up study published in 2021, patients who achieved culture conversion by Month 6 continued treatment for an additional 12 months, followed by off-treatment observation in order to assess the sustainability and durability of culture conversion. Following 12 months of post-conversion treatment, 63.1% (n= 41/65) of converters in the ALIS plus oral GBT arm and 30.0% (n=3/10) in the oral GBT alone arm achieved sustained conversion (P=0.0644). In the intention-to-treat population, which includes patients who did not culture convert, 18.3% (n=41/65) of patients in the ALIS plus oral GBT arm achieved sustained culture conversion compared with only 2.7% (n=3/10) in the oral GBT alone arm (P<0.0001). Three months following end of treatment, 55.4% (n=36/65) of ALIS plus oral GBT culture-converted patients also achieved durable culture conversion whereas no patients on oral GBT alone achieved durable culture conversion (P=0.0017). In the intention-to-treat population, 16.1% (n=36/224) of all patients on ALIS plus oral GBT achieved durable culture conversion versus no patients treated with oral GBT alone (P<0.0001).9
Re-emergence of a MAC strain with an identical genotype to the strain identified on initiation of treatment may indicate relapse, particularly if this occurs within the first 8 months of treatment. At the end of treatment, only 7.7% (n=5/65) of patients on ALIS plus oral GBT had relapsed compared with 30% (n=3/10) of patients on oral GBT alone. In contrast, recurrence of MAC after 8 months of treatment may indicate reinfection, in which MAC has been reacquired from the environment, and is treated as a new MAC infection. In total, 4.6% (n=3/65) of patients on ALIS plus oral GBT were reinfected with MAC, compared with 10% (n=1/10) of patients on oral GBT alone.9
Treatment emergent adverse events (TEAEs) occurred mainly in the first 8 months of treatment and were mainly respiratory. Respiratory TEAEs were reported more frequently in the ALIS plus oral GBT arm and included dysphonia (61.5%), cough (41.5%), dyspnoea (21.5%), and haemoptysis (20.0%). Nephrotoxicity TEAEs were balanced between the two arms (n=2 in both arms) and ototoxicity-related TEAEs in the ALIS plus GBT arm were primarily tinnitus (10.8%) and dizziness (7.7%). Only four patients discontinued treatment because of TEAEs in the ALIS plus oral GBT converter arm. Three patients who achieved culture conversion died, all in the oral GBT alone arm.9
The CONVERT study showed that the addition of ALIS to oral GBT significantly increased the likelihood of culture conversion by Month 6 compared with oral GBT alone, providing the first evidence in a randomised trial in addition to the Phase II study of efficacy against treatment-refractory MAC-PD (Figure 1).8 In addition, culture conversion in patients treated with ALIS in addition to oral GBT was generally sustained and durable with low risk of relapse (Figure 1).9 Long-term exposure to ALIS did not present any new safety concerns, with most TEAEs consistent with administration of an inhaled add-on antibiotic and generally occurring in the first 8 months of treatment.9 Overall, these results highlight the clinical utility of ALIS in the management of patients with refractory MAC-PD.
Figure 1. Proportion of patients achieving culture conversion by the first month of conversion.8,9
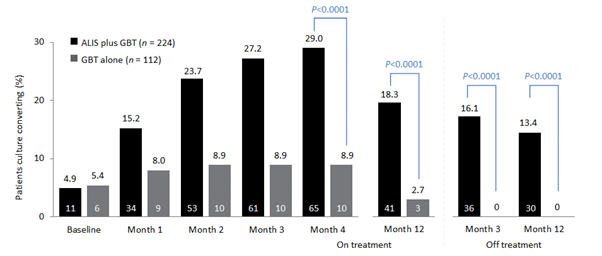
Month 4 was the last time point at which the first of three negative sputum cultures could be achieved for a patient to be considered a converter at Month 6.
ALIS, amikacin liposomal inhalation suspension; GBT, guideline-based therapy.
References:
- Griffith DE, Aksamit TR. Therapy of refractory nontuberculous mycobacterial lung disease. Curr Opin Infect Dis 2012;25:218–27.
- Jo K-W, Kim S, Lee JY, Lee SD, Kim WS, Kim DS, et al. Treatment outcomes of refractory MAC pulmonary disease treated with drugs with unclear efficacy. J Infect Chemother 2014;20:602–6.
- Rose SJ, Neville ME, Gupta R, Bermudez LE. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS ONE 2014:9:e108703.
- Malinin V, Neville M, Eagle G, Gupta R, Perkins WR. Pulmonary deposition and elimination of liposomal amikacin for inhalation and effect on macrophage function after administration in rats. Antimicrob Agents Chemother 2016;60:6540–9.
- Zhang J, Leifer F, Rose S, Chun DY, Thaisz J, Herr T, et al. amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol 2018;9:915.
- Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020;56:2000535.
- Olivier KN, Griffith DE, Eagle G, McGinnis JP 2nd, Micioni L, Liu K, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 2017;195:814–23.
- Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium aviumcomplex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med 2018;198:1559–69.
- Griffith DE, Thomson R, Flume PA, Aksamit TR, Field SK, Addrizzo-Harris DJ, et al. Amikacin liposome inhalation suspension for refractory Mycobacterium avium complex lung disease: sustainability and durability of culture conversion and safety of long-term exposure. Chest 2021; https://doi.org/10.1016/j.chest.2021.03.070
![]()
Medical writing and editorial support was provided by Highfield, Oxford, UK. This support was sponsored by Insmed.
Thank you for registering your interest
Sorry, an error has occurred: